Expression of GADS enhances FLT3-induced mitogenic signaling
- PMID: 26895103
- PMCID: PMC4924701
- DOI: 10.18632/oncotarget.7415
Expression of GADS enhances FLT3-induced mitogenic signaling
Abstract
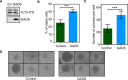
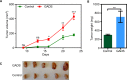
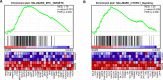
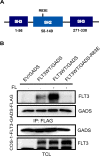
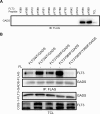
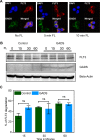
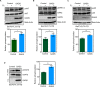
GADS is a member of a family of SH2 and SH3 domain-containing adaptors that functions in tyrosine kinase-mediated signaling cascades. Its expression is largely restricted to hematopoietic tissues and cell lines. Therefore, GADS is mainly involved in leukocyte-specific protein tyrosine kinase signaling. GADS is known to interact with tyrosine-phosphorylated SHC, BCR-ABL and KIT. The SH2 domain of GADS has a similar binding specificity to that of GRB2 but its SH3 domain displays a different binding specificity, and thus it is involved in other downstream signaling pathways than GRB2. In the present study, we examined the role of GADS in FLT3 signaling. FLT3 is a type III receptor tyrosine kinase, which is mutated in more than 30% of acute myeloid leukemia (AML) and the most common mutations is the internal tandem duplication (ITD) mutations. We observed that expression of GADS enhanced oncogenic FLT3-ITD-induced cell proliferation and colony formation in vitro. In a mouse xenograft model, GADS accelerated FLT3-ITD-dependent tumor formation. Furthermore, expression of GADS induced a transcriptional program leading to upregulation of MYC and mTORC1 target genes. GADS localizes to the cell membrane and strongly binds to ligand-stimulated wild-type FLT3 or is constitutively associated with the oncogenic mutant FLT3-ITD. We mapped the binding sites in FLT3 to pY955 and pY969 which overlaps with the GRB2 binding sites. Expression of GADS enhanced FLT3-mediated phosphorylation of AKT, ERK1/2, p38 and STAT5. Taken together, our data suggests that GADS is an important downstream component of FLT3 signaling and expression of GADS potentiates FLT3-mediated mitogenic signaling.
Keywords: AML; FLT3-ITD; GRAP2; RTK; STAT5.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kabir NN, Rönnstrand L, Kazi JU. FLT3 mutations in patients with childhood acute lymphoblastic leukemia (ALL) Med Oncol. 2013;30:462. - PubMed
-
- Masson K, Rönnstrand L. Oncogenic signaling from the hematopoietic growth factor receptors c-Kit and Flt3. Cell Signal. 2009;21:1717–1726. - PubMed
-
- Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, Hollingsworth LT, Picha KS, McKenna HJ, Splett RR, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75:1157–1167. - PubMed
-
- Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckmann MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–2801. - PubMed
-
- Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, Kastelein R, Hudak S, Wagner J, Mattson J, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368:643–648. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

