Immunisation With Immunodominant Linear B Cell Epitopes Vaccine of Manganese Transport Protein C Confers Protection against Staphylococcus aureus Infection
- PMID: 26895191
- PMCID: PMC4764517
- DOI: 10.1371/journal.pone.0149638
Immunisation With Immunodominant Linear B Cell Epitopes Vaccine of Manganese Transport Protein C Confers Protection against Staphylococcus aureus Infection
Abstract
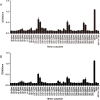
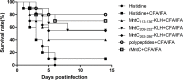
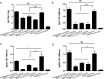
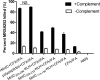
Vaccination strategies for Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA) infections have attracted much research attention. Recent efforts have been made to select manganese transport protein C, or manganese binding surface lipoprotein C (MntC), which is a metal ion associated with pathogen nutrition uptake, as potential candidates for an S. aureus vaccine. Although protective humoral immune responses to MntC are well-characterised, much less is known about detailed MntC-specific B cell epitope mapping and particularly epitope vaccines, which are less-time consuming and more convenient. In this study, we generated a recombinant protein rMntC which induced strong antibody response when used for immunisation with CFA/IFA adjuvant. On the basis of the results, linear B cell epitopes within MntC were finely mapped using a series of overlapping synthetic peptides. Further studies indicate that MntC113-136, MntC209-232, and MntC263-286 might be the original linear B-cell immune dominant epitope of MntC, furthermore, three-dimensional (3-d) crystal structure results indicate that the three immunodominant epitopes were displayed on the surface of the MntC antigen. On the basis of immunodominant MntC113-136, MntC209-232, and MntC263-286 peptides, the epitope vaccine for S. aureus induces a high antibody level which is biased to TH2 and provides effective immune protection and strong opsonophagocytic killing activity in vitro against MRSA infection. In summary, the study provides strong proof of the optimisation of MRSA B cell epitope vaccine designs and their use, which was based on the MntC antigen in the development of an MRSA vaccine.
Conflict of interest statement
Figures


References
-
- Cosseron-Zerbib M, Roque Afonso AM, Naas T, Durand P, Meyer L, Costa Y, et al. A control programme for MRSA (methicillin-resistant Staphylococcus aureus) containment in a paediatric intensive care unit: evaluation and impact on infections caused by other micro-organisms. The Journal of hospital infection. 1998;40(3):225–35. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

