Sequence-Dependent Fluorescence of Cy3- and Cy5-Labeled Double-Stranded DNA
- PMID: 26895222
- PMCID: PMC4796863
- DOI: 10.1021/acs.bioconjchem.6b00053
Sequence-Dependent Fluorescence of Cy3- and Cy5-Labeled Double-Stranded DNA
Abstract
The fluorescent intensity of Cy3 and Cy5 dyes is strongly dependent on the nucleobase sequence of the labeled oligonucleotides. Sequence-dependent fluorescence may significantly influence the data obtained from many common experimental methods based on fluorescence detection of nucleic acids, such as sequencing, PCR, FRET, and FISH. To quantify sequence dependent fluorescence, we have measured the fluorescence intensity of Cy3 and Cy5 bound to the 5' end of all 1024 possible double-stranded DNA 5mers. The fluorescence intensity was also determined for these dyes bound to the 5' end of fixed-sequence double-stranded DNA with a variable sequence 3' overhang adjacent to the dye. The labeled DNA oligonucleotides were made using light-directed, in situ microarray synthesis. The results indicate that the fluorescence intensity of both dyes is sensitive to all five bases or base pairs, that the sequence dependence is stronger for double- (vs single-) stranded DNA, and that the dyes are sensitive to both the adjacent dsDNA sequence and the 3'-ssDNA overhang. Purine-rich sequences result in higher fluorescence. The results can be used to estimate measurement error in experiments with fluorescent-labeled DNA, as well as to optimize the fluorescent signal by considering the nucleobase environment of the labeling cyanine dye.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




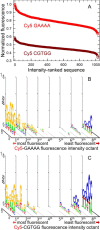
Similar articles
-
Comparison of the sequence-dependent fluorescence of the cyanine dyes Cy3, Cy5, DyLight DY547 and DyLight DY647 on single-stranded DNA.PLoS One. 2014 Jan 15;9(1):e85605. doi: 10.1371/journal.pone.0085605. eCollection 2014. PLoS One. 2014. PMID: 24454899 Free PMC article.
-
Sequence-dependence of Cy3 and Cy5 dyes in 3' terminally-labeled single-stranded DNA.Sci Rep. 2022 Aug 31;12(1):14803. doi: 10.1038/s41598-022-19069-9. Sci Rep. 2022. PMID: 36045146 Free PMC article.
-
Sequence-dependent fluorescence of cyanine dyes on microarrays.PLoS One. 2011;6(7):e22177. doi: 10.1371/journal.pone.0022177. Epub 2011 Jul 25. PLoS One. 2011. PMID: 21799789 Free PMC article.
-
Fluorescence resonance energy transfer (FRET) and competing processes in donor-acceptor substituted DNA strands: a comparative study of ensemble and single-molecule data.J Biotechnol. 2002 Jan;82(3):211-31. doi: 10.1016/s1389-0352(01)00039-3. J Biotechnol. 2002. PMID: 11999691 Review.
-
Symmetric cyanine dyes for detecting nucleic acids.Biotech Histochem. 2008 Jun;83(3-4):131-45. doi: 10.1080/10520290802383684. Biotech Histochem. 2008. PMID: 18802811 Review.
Cited by
-
Optical and theoretical study of strand recognition by nucleic acid probes.Commun Chem. 2020 Aug 11;3(1):111. doi: 10.1038/s42004-020-00362-5. Commun Chem. 2020. PMID: 36703315 Free PMC article.
-
Replication of [AT/TA]25 Microsatellite Sequences by Human DNA Polymerase δ Holoenzymes Is Dependent on dNTP and RPA Levels.Biochemistry. 2024 Apr 16;63(8):969-983. doi: 10.1021/acs.biochem.4c00006. Epub 2024 Mar 26. Biochemistry. 2024. PMID: 38623046 Free PMC article.
-
Unraveling biomolecular interactions: a comprehensive review of the electromobility shift assay.Photochem Photobiol Sci. 2025 Sep 11. doi: 10.1007/s43630-025-00773-0. Online ahead of print. Photochem Photobiol Sci. 2025. PMID: 40931273 Review.
-
Spectroscopic Characterization of Thiacarbocyanine Dye Molecules Adsorbed on Hexagonal Boron Nitride: a Time-Resolved Study.ACS Omega. 2023 Sep 20;8(39):35638-35652. doi: 10.1021/acsomega.3c02020. eCollection 2023 Oct 3. ACS Omega. 2023. PMID: 37810698 Free PMC article.
-
Mimicking a Cellular Crowding Environment for Enzyme-Free Paper-Based Nucleic Acid Tests at the Point of Care.ACS Sens. 2024 Oct 25;9(10):5069-5080. doi: 10.1021/acssensors.4c00539. Epub 2024 Sep 30. ACS Sens. 2024. PMID: 39344686 Free PMC article.
References
-
- Brauns E. B.; Murphy C. J.; Berg M. A. (1998) Local dynamics in DNA by temperature-dependent Stokes shifts of an intercalated dye. J. Am. Chem. Soc. 120, 2449–2456. 10.1021/ja973207+. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

