NPAS2 Compensates for Loss of CLOCK in Peripheral Circadian Oscillators
- PMID: 26895328
- PMCID: PMC4760943
- DOI: 10.1371/journal.pgen.1005882
NPAS2 Compensates for Loss of CLOCK in Peripheral Circadian Oscillators
Abstract
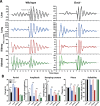
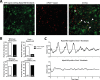
Heterodimers of CLOCK and BMAL1 are the major transcriptional activators of the mammalian circadian clock. Because the paralog NPAS2 can substitute for CLOCK in the suprachiasmatic nucleus (SCN), the master circadian pacemaker, CLOCK-deficient mice maintain circadian rhythms in behavior and in tissues in vivo. However, when isolated from the SCN, CLOCK-deficient peripheral tissues are reportedly arrhythmic, suggesting a fundamental difference in circadian clock function between SCN and peripheral tissues. Surprisingly, however, using luminometry and single-cell bioluminescence imaging of PER2 expression, we now find that CLOCK-deficient dispersed SCN neurons and peripheral cells exhibit similarly stable, autonomous circadian rhythms in vitro. In CLOCK-deficient fibroblasts, knockdown of Npas2 leads to arrhythmicity, suggesting that NPAS2 can compensate for loss of CLOCK in peripheral cells as well as in SCN. Our data overturn the notion of an SCN-specific role for NPAS2 in the molecular circadian clock, and instead indicate that, at the cellular level, the core loops of SCN neuron and peripheral cell circadian clocks are fundamentally similar.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Advanced light-entrained activity onsets and restored free-running suprachiasmatic nucleus circadian rhythms in per2/dec mutant mice.Chronobiol Int. 2011 Nov;28(9):737-50. doi: 10.3109/07420528.2011.607374. Chronobiol Int. 2011. PMID: 22080784
-
Differential maturation of circadian rhythms in clock gene proteins in the suprachiasmatic nucleus and the pars tuberalis during mouse ontogeny.Eur J Neurosci. 2009 Feb;29(3):477-89. doi: 10.1111/j.1460-9568.2008.06605.x. Eur J Neurosci. 2009. PMID: 19222558 Free PMC article.
-
CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock.Nat Neurosci. 2007 May;10(5):543-5. doi: 10.1038/nn1884. Epub 2007 Apr 8. Nat Neurosci. 2007. PMID: 17417633 Free PMC article.
-
The Retinal Circadian Clock and Photoreceptor Viability.Adv Exp Med Biol. 2018;1074:345-350. doi: 10.1007/978-3-319-75402-4_42. Adv Exp Med Biol. 2018. PMID: 29721962 Free PMC article. Review.
-
Development of the mammalian circadian clock.Eur J Neurosci. 2020 Jan;51(1):182-193. doi: 10.1111/ejn.14318. Epub 2019 Jan 30. Eur J Neurosci. 2020. PMID: 30589961 Review.
Cited by
-
Role of the Circadian Gas-Responsive Hemeprotein NPAS2 in Physiology and Pathology.Biology (Basel). 2023 Oct 22;12(10):1354. doi: 10.3390/biology12101354. Biology (Basel). 2023. PMID: 37887064 Free PMC article. Review.
-
High-throughput measurement of fibroblast rhythms reveals genetic heritability of circadian phenotypes in diversity outbred mice and their founder strains.Sci Rep. 2021 Jan 28;11(1):2573. doi: 10.1038/s41598-021-82069-8. Sci Rep. 2021. PMID: 33510298 Free PMC article.
-
Involvement of posttranscriptional regulation of Clock in the emergence of circadian clock oscillation during mouse development.Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):E7479-E7488. doi: 10.1073/pnas.1703170114. Epub 2017 Aug 21. Proc Natl Acad Sci U S A. 2017. PMID: 28827343 Free PMC article.
-
Non-transcriptional processes in circadian rhythm generation.Curr Opin Physiol. 2018 Oct;5:117-132. doi: 10.1016/j.cophys.2018.10.003. Curr Opin Physiol. 2018. PMID: 30596188 Free PMC article. Review.
-
Identification and characterization of the CDK1-BMAL1-UHRF1 pathway driving tumor progression.iScience. 2023 Mar 31;26(4):106544. doi: 10.1016/j.isci.2023.106544. eCollection 2023 Apr 21. iScience. 2023. PMID: 37123229 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

