Tumoral indoleamine 2, 3-dioxygenase 1 is regulated by monocytes and T lymphocytes collaboration in hepatocellular carcinoma
- PMID: 26895379
- PMCID: PMC4924751
- DOI: 10.18632/oncotarget.7438
Tumoral indoleamine 2, 3-dioxygenase 1 is regulated by monocytes and T lymphocytes collaboration in hepatocellular carcinoma
Abstract
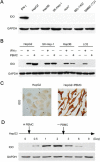
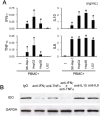
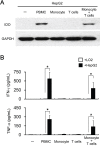
Indoleamine 2, 3-Dioxygenase 1 (IDO1) in cancer cells plays a critical role in tumor immunosuppression. However, the precise mechanisms regulating tumoral IDO1 expression in tumor milieus remain unclear. Here, we reported that IDO1 expression in tumor cells of hepatocelluar carcinomas (HCC), displayed a discrete rather than uniform pattern. In vitro culture, human hepatoma cell lines did not constitutively express IDO1. Interestingly, co-culture with peripheral blood mononuclear cells (PBMC) significantly induced and maintained IDO1 expression in these tumor cells, predominantly through IFN-γ. Mechanistically, we showed that IDO1 expression in tumor cells was only induced when co-cultured with both T lymphocytes and monocytes. Moreover, the cooperation between T lymphocytes and monocytes played an indispensable role on the tumoral IDO1 expression in immunocompromised mice. Taken together, our data supported the notion that IDO1 expression in tumor cells might serve as a counter-regulatory mechanism regulated by immune system, and provided new insights into the collaborative action of different inflammatory cells in tumor immunosuppression.
Keywords: IDO1; T cells; monocytes; tumor cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. - PubMed
-
- Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, Herve C, Gutierrez-Roelens I, Marbaix E, Sempoux C, Van den Eynde BJ. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer immunology research. 2015;3:161–172. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

