In Vitro Effects of the Endocrine Disruptor p,p'-DDT on Human Follitropin Receptor
- PMID: 26895433
- PMCID: PMC4937862
- DOI: 10.1289/ehp.1510006
In Vitro Effects of the Endocrine Disruptor p,p'-DDT on Human Follitropin Receptor
Abstract
Background: 1-chloro-4-[2,2,2-trichloro-1-(4-chlorophenyl)ethyl]benzene (p,p'-DDT) is a persistent environmental endocrine disruptor (ED). Several studies have shown an association between p,p'-DDT exposure and reproductive abnormalities.
Objectives: To investigate the putative effects of p,p'-DDT on the human follitropin receptor (FSHR) function.
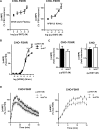
Methods and results: We used Chinese hamster ovary (CHO) cells stably expressing human FSHR to investigate the impact of p,p'-DDT on FSHR activity and its interaction with the receptor. At a concentration of 5 μM p,p'-DDT increased the maximum response of the FSHR to follitropin by 32 ± 7.45%. However, 5 μM p,p'-DDT decreased the basal activity and did not influence the maximal response of the closely related LH/hCG receptor to human chorionic gonadotropin (hCG). The potentiating effect of p,p'-DDT was specific for the FSHR. Moreover, in cells that did not express FSHR, p,p'-DDT had no effect on cAMP response. Thus, the potentiating effect of p,p'-DDT was dependent on the FSHR. In addition, p,p'-DDT increased the sensitivity of FSHR to hCG and to a low molecular weight agonist of the FSHR, 3-((5methyl)-2-(4-benzyloxy-phenyl)-5-{[2-[3-ethoxy-4-methoxy-phenyl)-ethylcarbamoyl]-methyl}-4-oxo-thiazolidin-3-yl)-benzamide (16a). Basal activity in response to p,p'-DDT and potentiation of the FSHR response to FSH by p,p'-DDT varied among FSHR mutants with altered transmembrane domains (TMDs), consistent with an effect of p,p'-DDT via TMD binding. This finding was corroborated by the results of simultaneously docking p,p'-DDT and 16a into the FSHR transmembrane bundle.
Conclusion: p,p'-DDT acted as a positive allosteric modulator of the FSHR in our experimental model. These findings suggest that G protein-coupled receptors are additional targets of endocrine disruptors.
Citation: Munier M, Grouleff J, Gourdin L, Fauchard M, Chantreau V, Henrion D, Coutant R, Schiøtt B, Chabbert M, Rodien P. 2016. In vitro effects of the endocrine disruptor p,p'-DDT on human follitropin receptor. Environ Health Perspect 124:991-999; http://dx.doi.org/10.1289/ehp.1510006.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures




References
-
- Alewijnse AE, Smit MJ, Rodriguez Pena MS, Verzijl D, Timmerman H, Leurs R. Modulation of forskolin-mediated adenylyl cyclase activation by constitutively active GS-coupled receptors. FEBS Lett. 1997;419:171–174. - PubMed
-
- Bergman Å, Heindel JJ, Jobling S, Kidd KA, Zoeller RT, eds. Geneva: World Health Organization; 2013. State of the Science of Endocrine Disrupting Chemicals 2012. Available: http://www.who.int/iris/bitstream/10665/78101/1/9789241505031_eng.pdf?ua=1 [accessed 31 May 2016]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

