Redox-Annulation of Cyclic Amines and β-Ketoaldehydes
- PMID: 26895555
- PMCID: PMC4782176
- DOI: 10.1021/acs.orglett.6b00151
Redox-Annulation of Cyclic Amines and β-Ketoaldehydes
Abstract
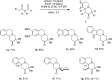
Benzo[a]quinolizine-2-one derivatives are readily assembled from 1,2,3,4-tetrahydroisoquinoline and β-ketoaldehydes by means of a new intramolecular redox-Mannich process. These reactions are promoted by simple acetic acid and are thought to involve azomethine ylides as reactive intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
-
For selected recent syntheses of the compounds shown in Scheme 1, see:
- Itoh T.; Yokoya M.; Miyauchi K.; Nagata K.; Ohsawa A. Org. Lett. 2006, 8, 1533.10.1021/ol0530575. - DOI - PubMed
- Rishel M. J.; Amarasinghe K. K. D.; Dinn S. R.; Johnson B. F. J. Org. Chem. 2009, 74, 4001.10.1021/jo900480n. - DOI - PubMed
- Paek S.-M.; Kim N.-J.; Shin D.; Jung J.-K.; Jung J.-W.; Chang D.-J.; Moon H.; Suh Y.-G. Chem. - Eur. J. 2010, 16, 4623.10.1002/chem.200902591. - DOI - PubMed
- Son Y.-W.; Kwon T.-H.; Lee J.-K.; Pae A.-N.; Lee J.-Y.; Cho Y.-S.; Min S.-J. Org. Lett. 2011, 13, 6500.10.1021/ol202792q. - DOI - PubMed
- Sun X.; Ma D. Chem. - Asian J. 2011, 6, 2158.10.1002/asia.201100219. - DOI - PubMed
- Zhang W.; Bah J.; Wohlfarth A.; Franzén J. Chem. - Eur. J. 2011, 17, 13814.10.1002/chem.201102012. - DOI - PubMed
- Johannes M.; Altmann K.-H. Org. Lett. 2012, 14, 3752.10.1021/ol301612q. - DOI - PubMed
- Lin S.; Deiana L.; Tseggai A.; Córdova A. Eur. J. Org. Chem. 2012, 2012, 398.10.1002/ejoc.201101296. - DOI
- Orgren L. R.; Maverick E. E.; Marvin C. C. J. Org. Chem. 2015, 80, 12635.10.1021/acs.joc.5b02199. - DOI - PMC - PubMed
-
-
-
For selected syntheses of benzo[a]quinolizine-2-one derivatives, see:
- Battersby A. R.; Openshaw H. T.; Wood H. C. S. J. Chem. Soc. 1953, 2463.10.1039/jr9530002463. - DOI
- Itoh N.; Sugasawa S. J. Org. Chem. 1959, 24, 2042.10.1021/jo01094a619. - DOI
- Openshaw H. T.; Whittaker N. J. Chem. Soc. 1963, 1449.10.1039/jr9630001449. - DOI
- Ninomiya I.; Tada Y.; Kiguchi T.; Yamamoto O.; Naito T. J. Chem. Soc., Perkin Trans. 1 1984, 2035.10.1039/p19840002035. - DOI
- Brandi A.; Garro S.; Guarna A.; Goti A.; Cordero F.; De Sarlo F. J. Org. Chem. 1988, 53, 2430.10.1021/jo00246a008. - DOI
- Guiles J. W.; Meyers A. I. J. Org. Chem. 1991, 56, 6873.10.1021/jo00024a032. - DOI
- Flick A. C.; Padwa A. Tetrahedron Lett. 2008, 49, 5739.10.1016/j.tetlet.2008.07.109. - DOI
- Zheng P.; Lieberman B. P.; Choi S. R.; Plöessl K.; Kung H. F. Bioorg. Med. Chem. Lett. 2011, 21, 3435.10.1016/j.bmcl.2011.03.113. - DOI - PubMed
- Jones K. M.; Karier P.; Klussmann M. ChemCatChem 2012, 4, 51.10.1002/cctc.201100324. - DOI
- Zhang G.; Wang S.; Ma Y.; Kong W.; Wang R. Adv. Synth. Catal. 2013, 355, 874.10.1002/adsc.201200731. - DOI
-
-
-
Selected reviews on amine C–H functionalization, including redox-neutral approaches:
- Murahashi S.-I. Angew. Chem., Int. Ed. Engl. 1995, 34, 2443.10.1002/anie.199524431. - DOI
- Matyus P.; Elias O.; Tapolcsanyi P.; Polonka-Balint A.; Halasz-Dajka B. Synthesis 2006, 2006, 2625.10.1055/s-2006-942490. - DOI
- Campos K. R. Chem. Soc. Rev. 2007, 36, 1069.10.1039/b607547a. - DOI - PubMed
- Murahashi S.-I.; Zhang D. Chem. Soc. Rev. 2008, 37, 1490.10.1039/b706709g. - DOI - PubMed
- Li C.-J. Acc. Chem. Res. 2009, 42, 335.10.1021/ar800164n. - DOI - PubMed
- Jazzar R.; Hitce J.; Renaudat A.; Sofack-Kreutzer J.; Baudoin O. Chem. - Eur. J. 2010, 16, 2654.10.1002/chem.200902374. - DOI - PubMed
- Yeung C. S.; Dong V. M. Chem. Rev. 2011, 111, 1215.10.1021/cr100280d. - DOI - PubMed
- Pan S. C. Beilstein J. Org. Chem. 2012, 8, 1374.10.3762/bjoc.8.159. - DOI - PMC - PubMed
- Mitchell E. A.; Peschiulli A.; Lefevre N.; Meerpoel L.; Maes B. U. W. Chem. - Eur. J. 2012, 18, 10092.10.1002/chem.201201539. - DOI - PubMed
- Zhang C.; Tang C.; Jiao N. Chem. Soc. Rev. 2012, 41, 3464.10.1039/c2cs15323h. - DOI - PubMed
- Jones K. M.; Klussmann M. Synlett 2012, 2012, 159.10.1055/s-0031-1290117. - DOI
- Peng B.; Maulide N. Chem. - Eur. J. 2013, 19, 13274.10.1002/chem.201301522. - DOI - PubMed
- Platonova A. Y.; Glukhareva T. V.; Zimovets O. A.; Morzherin Y. Y. Chem. Heterocycl. Compd. 2013, 49, 357.10.1007/s10593-013-1257-6. - DOI
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322.10.1021/cr300503r. - DOI - PMC - PubMed
- Girard S. A.; Knauber T.; Li C.-J. Angew. Chem., Int. Ed. 2014, 53, 74.10.1002/anie.201304268. - DOI - PubMed
- Haibach M. C.; Seidel D. Angew. Chem., Int. Ed. 2014, 53, 5010.10.1002/anie.201306489. - DOI - PubMed
- Wang L.; Xiao J. Adv. Synth. Catal. 2014, 356, 1137.10.1002/adsc.201301153. - DOI
- Vo C.-V. T.; Bode J. W. J. Org. Chem. 2014, 79, 2809.10.1021/jo5001252. - DOI - PubMed
- Seidel D. Org. Chem. Front. 2014, 1, 426.10.1039/c4qo00022f. - DOI - PMC - PubMed
- Qin Y.; Lv J.; Luo S. Tetrahedron Lett. 2014, 55, 551.10.1016/j.tetlet.2013.11.051. - DOI
- Beatty J. W.; Stephenson C. R. J. Acc. Chem. Res. 2015, 48, 1474.10.1021/acs.accounts.5b00068. - DOI - PMC - PubMed
-
-
-
Selected reviews on other types of redox-neutral transformations:
- Burns N. Z.; Baran P. S.; Hoffmann R. W. Angew. Chem., Int. Ed. 2009, 48, 2854.10.1002/anie.200806086. - DOI - PubMed
- Mahatthananchai J.; Bode J. W. Acc. Chem. Res. 2014, 47, 696.10.1021/ar400239v. - DOI - PubMed
- Ketcham J. M.; Shin I.; Montgomery T. P.; Krische M. J. Angew. Chem., Int. Ed. 2014, 53, 9142.10.1002/anie.201403873. - DOI - PMC - PubMed
- Huang H.; Ji X.; Wu W.; Jiang H. Chem. Soc. Rev. 2015, 44, 1155.10.1039/C4CS00288A. - DOI - PubMed
-
-
- Seidel D. Acc. Chem. Res. 2015, 48, 317.10.1021/ar5003768. - DOI - PMC - PubMed
-
Selected examples from our lab:
- Zhang C.; De C. K.; Mal R.; Seidel D. J. Am. Chem. Soc. 2008, 130, 416.10.1021/ja077473r. - DOI - PubMed
- Ma L.; Chen W.; Seidel D. J. Am. Chem. Soc. 2012, 134, 15305.10.1021/ja308009g. - DOI - PubMed
- Das D.; Sun A. X.; Seidel D. Angew. Chem., Int. Ed. 2013, 52, 3765.10.1002/anie.201300021. - DOI - PMC - PubMed
- Dieckmann A.; Richers M. T.; Platonova A. Y.; Zhang C.; Seidel D.; Houk K. N. J. Org. Chem. 2013, 78, 4132.10.1021/jo400483h. - DOI - PMC - PubMed
- Chen W.; Seidel D. Org. Lett. 2014, 16, 3158.10.1021/ol501365j. - DOI - PMC - PubMed
- Richers M. T.; Breugst M.; Platonova A. Y.; Ullrich A.; Dieckmann A.; Houk K. N.; Seidel D. J. Am. Chem. Soc. 2014, 136, 6123.10.1021/ja501988b. - DOI - PMC - PubMed
- Kang Y.; Richers M. T.; Sawicki C. H.; Seidel D. Chem. Commun. 2015, 51, 10648.10.1039/C5CC03390J. - DOI - PMC - PubMed
- Ma L.; Seidel D. Chem. - Eur. J. 2015, 21, 12908.10.1002/chem.201501667. - DOI - PMC - PubMed
- Kang Y.; Chen W.; Breugst M.; Seidel D. J. Org. Chem. 2015, 80, 9628.10.1021/acs.joc.5b01384. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

