Feline panleukopenia virus in cerebral neurons of young and adult cats
- PMID: 26895627
- PMCID: PMC4759964
- DOI: 10.1186/s12917-016-0657-0
Feline panleukopenia virus in cerebral neurons of young and adult cats
Abstract
Background: Perinatal infections with feline panleukopenia virus (FPV) have long been known to be associated with cerebellar hypoplasia in kittens due to productive infection of dividing neuroblasts. FPV, like other parvoviruses, requires dividing cells to replicate which explains the usual tropism of the virus for the digestive tract, lymphoid tissues and bone marrow in older animals.
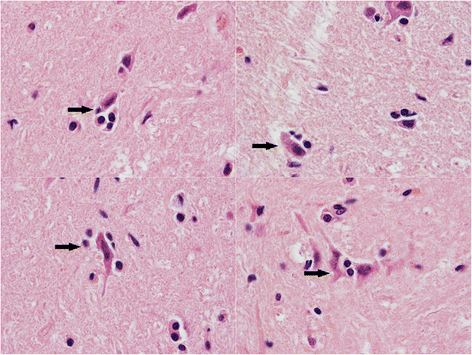
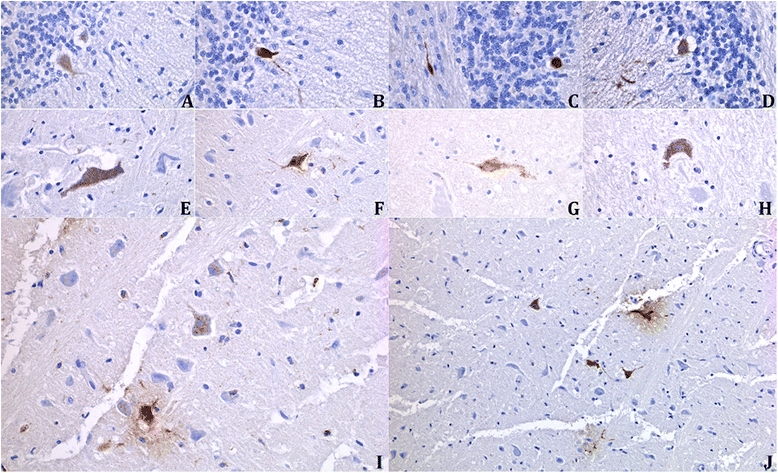
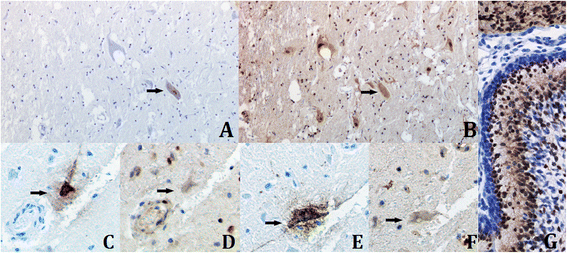
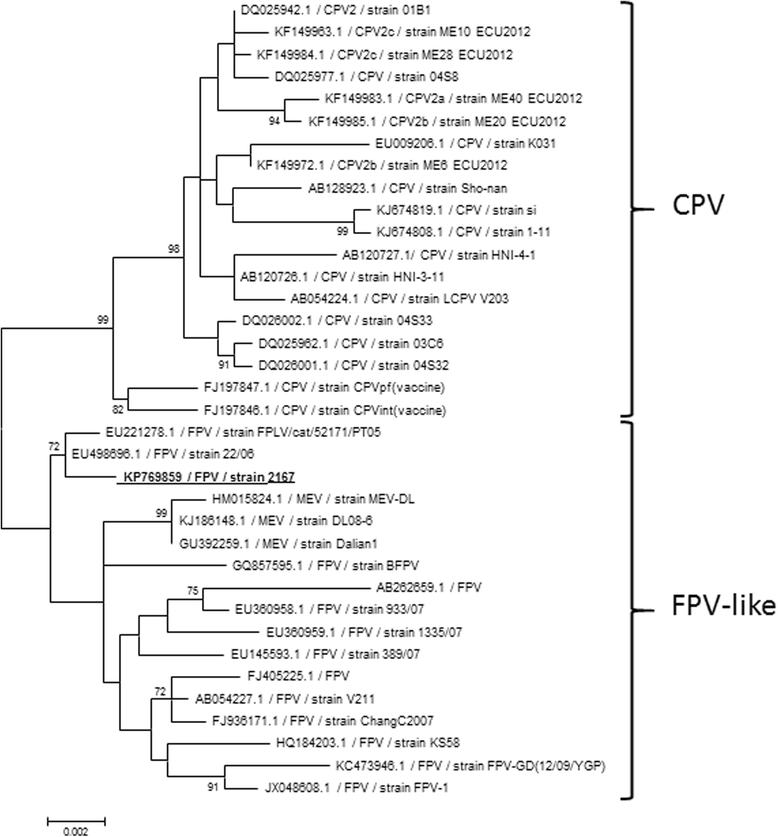
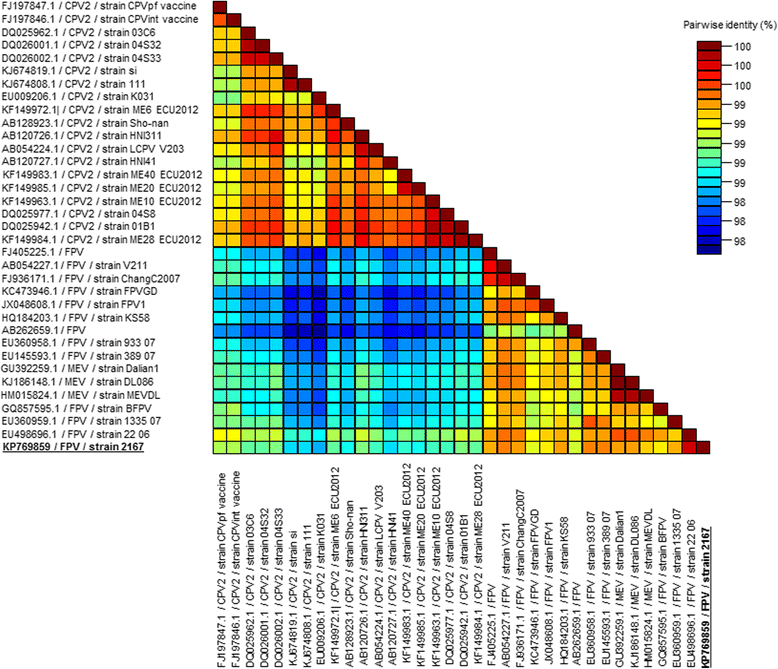
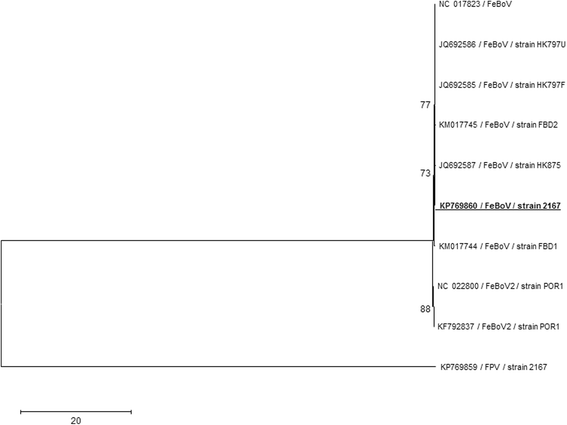
Results: In this study, the necropsy and histopathological analyses of a series of 28 cats which died from parvovirus infection in 2013 were performed. Infections were confirmed by real time PCR and immunohistochemistry in several organs. Strikingly, while none of these cats showed cerebellar atrophy or cerebellar positive immunostaining, some of them, including one adult, showed a bright positive immunostaining for viral antigens in cerebral neurons (diencephalon). Furthermore, infected neurons were negative by immunostaining for p27(Kip1), a cell cycle regulatory protein, while neighboring, uninfected, neurons were positive, suggesting a possible re-entry of infected neurons into the mitotic cycle. Next-Generation Sequencing and PCR analyses showed that the virus infecting cat brains was FPV and presented a unique substitution in NS1 protein sequence. Given the role played by this protein in the control of cell cycle and apoptosis in other parvoviral species, it is tempting to hypothesize that a cause-to-effect between this NS1 mutation and the capacity of this FPV strain to infect neurons in adult cats might exist.
Conclusions: This study provides the first evidence of infection of cerebral neurons by feline panleukopenia virus in cats, including an adult. A possible re-entry into the cell cycle by infected neurons has been observed. A mutation in the NS1 protein sequence of the FPV strain involved could be related to its unusual cellular tropism. Further research is needed to clarify this point.
Figures
Similar articles
-
Cell cycle S phase markers are expressed in cerebral neuron nuclei of cats infected by the Feline Panleukopenia Virus.Cell Cycle. 2016 Dec 16;15(24):3482-3489. doi: 10.1080/15384101.2016.1249546. Epub 2016 Nov 10. Cell Cycle. 2016. PMID: 27830988 Free PMC article.
-
Neuronal Vacuolization in Feline Panleukopenia Virus Infection.Vet Pathol. 2018 Mar;55(2):294-297. doi: 10.1177/0300985817738096. Epub 2017 Nov 20. Vet Pathol. 2018. PMID: 29157191
-
Identification of parvovirus in the bone marrow of eight cats.Aust Vet J. 2012 Apr;90(4):136-9. doi: 10.1111/j.1751-0813.2012.00899.x. Aust Vet J. 2012. PMID: 22443328
-
Feline parvovirus infection and associated diseases.Vet J. 2014 Aug;201(2):150-5. doi: 10.1016/j.tvjl.2014.05.027. Epub 2014 May 22. Vet J. 2014. PMID: 24923754 Review.
-
Feline Panleukopenia: A Re-emergent Disease.Vet Clin North Am Small Anim Pract. 2019 Jul;49(4):651-670. doi: 10.1016/j.cvsm.2019.02.006. Epub 2019 Apr 6. Vet Clin North Am Small Anim Pract. 2019. PMID: 30967253 Review.
Cited by
-
Simultaneous detection and differentiation of canine parvovirus and feline parvovirus by high resolution melting analysis.BMC Vet Res. 2019 May 10;15(1):141. doi: 10.1186/s12917-019-1898-5. BMC Vet Res. 2019. PMID: 31077252 Free PMC article.
-
Development and Application of an RPA-Based Rapid Point-of-Care Testing (POCT) Method for the Detection of Feline Panleukopenia Virus.Transbound Emerg Dis. 2024 Aug 24;2024:3680778. doi: 10.1155/2024/3680778. eCollection 2024. Transbound Emerg Dis. 2024. PMID: 40303162 Free PMC article.
-
Molecular and Pathological Investigations of Selected Viral Neuropathogens in Rabies-Negative Brains of Cats and Dogs Revealed Neurotropism of Carnivore Protoparvovirus-1.Front Vet Sci. 2021 Aug 19;8:710701. doi: 10.3389/fvets.2021.710701. eCollection 2021. Front Vet Sci. 2021. PMID: 34490401 Free PMC article.
-
Development and Application of nanoPCR Method for Detection of Feline Panleukopenia Virus.Vet Sci. 2023 Jul 6;10(7):440. doi: 10.3390/vetsci10070440. Vet Sci. 2023. PMID: 37505845 Free PMC article.
-
Simultaneous detection of feline parvovirus and feline bocavirus using SYBR Green I-based duplex real-time polymerase chain reaction.3 Biotech. 2021 Sep;11(9):400. doi: 10.1007/s13205-021-02947-w. Epub 2021 Aug 6. 3 Biotech. 2021. PMID: 34377624 Free PMC article.
References
-
- Siegl G, Bates RC, Berns KI, Carter BJ, Kelly DC, Kurstak E, Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23:61–73. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

