Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming
- PMID: 26895644
- PMCID: PMC4836113
- DOI: 10.1152/ajplung.00419.2015
Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming
Abstract
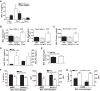
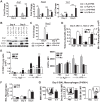
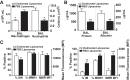
Despite intense investigation, acute respiratory distress syndrome (ARDS) remains an enormous clinical problem for which no specific therapies currently exist. In this study, we used intratracheal lipopolysaccharide or Pseudomonas bacteria administration to model experimental acute lung injury (ALI) and to further understand mediators of the resolution phase of ARDS. Recent work demonstrates macrophages transition from a predominant proinflammatory M1 phenotype during acute inflammation to an anti-inflammatory M2 phenotype with ALI resolution. We tested the hypothesis that IL-4, a potent inducer of M2-specific protein expression, would accelerate ALI resolution and lung repair through reprogramming of endogenous inflammatory macrophages. In fact, IL-4 treatment was found to offer dramatic benefits following delayed administration to mice subjected to experimental ALI, including increased survival, accelerated resolution of lung injury, and improved lung function. Expression of the M2 proteins Arg1, FIZZ1, and Ym1 was increased in lung tissues following IL-4 treatment, and among macrophages, FIZZ1 was most prominently upregulated in the interstitial subpopulation. A similar trend was observed for the expression of macrophage mannose receptor (MMR) and Dectin-1 on the surface of alveolar macrophages following IL-4 administration. Macrophage depletion or STAT6 deficiency abrogated the therapeutic effect of IL-4. Collectively, these data demonstrate that IL-4-mediated therapeutic macrophage reprogramming can accelerate resolution and lung repair despite delayed use following experimental ALI. IL-4 or other therapies that target late-phase, proresolution pathways may hold promise for the treatment of human ARDS.
Keywords: ARDS; acute lung injury; interleukin-4; macrophage; resolution.
Copyright © 2016 the American Physiological Society.
Figures





References
-
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury, and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000. - PubMed
-
- Aggarwal NR, Tsushima K, Eto Y, Tripathi A, Mandke P, Mock JR, Garibaldi BT, Singer BD, Sidhaye VK, Horton MR, King LS, D'Alessio FR. Immunological priming requires regulatory T cells and IL-10-producing macrophages to accelerate resolution from severe lung inflammation. J Immunol 192: 4453–4464, 2014. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

