Virus replicon particles expressing porcine reproductive and respiratory syndrome virus proteins elicit immune priming but do not confer protection from viremia in pigs
- PMID: 26895704
- PMCID: PMC4761149
- DOI: 10.1186/s13567-016-0318-0
Virus replicon particles expressing porcine reproductive and respiratory syndrome virus proteins elicit immune priming but do not confer protection from viremia in pigs
Abstract
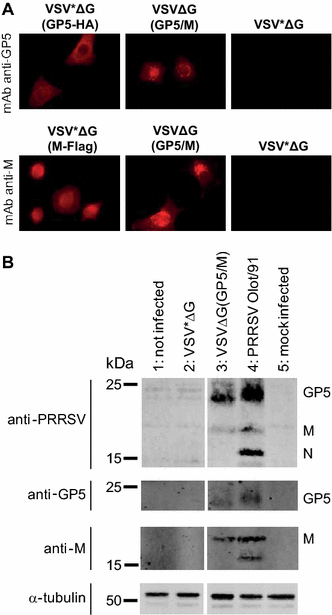
Porcine reproductive and respiratory syndrome virus (PRRSV) is the causative agent of one of the most devastating and economically significant viral disease of pigs worldwide. The vaccines currently available on the market elicit only limited protection. Recombinant vesicular stomatitis virus (VSV) replicon particles (VRP) have been used successfully to induce protection against influenza A virus (IAV) in chickens and bluetongue virus in sheep. In this study, VSV VRP expressing the PRRSV envelope proteins GP5, M, GP4, GP3, GP2 and the nucleocapsid protein N, individually or in combination, were generated and evaluated as a potential vector vaccine against PRRSV infection. High level expression of the recombinant PRRSV proteins was demonstrated in cell culture. However, none of the PRRSV antigens expressed from VRP, with the exception of the N protein, did induce any detectable antibody response in pigs before challenge infection with PRRSV. After challenge however, the antibody responses against GP5, GP4 and GP3 appeared in average 2 weeks earlier than in pigs vaccinated with the empty control VRP. No reduction of viremia was observed in the vaccinated group compared with the control group. When pigs were co-vaccinated with VRP expressing IAV antigens and VRP expressing PRRSV glycoproteins, only antibody responses to the IAV antigens were detectable. These data show that the VSV replicon vector can induce immune responses to heterologous proteins in pigs, but that the PRRSV envelope proteins expressed from VSV VRP are poorly immunogenic. Nevertheless, they prime the immune system for significantly earlier B-cell responses following PRRSV challenge infection.
Figures

References
-
- Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 2005;227:385–392. doi: 10.2460/javma.2005.227.385. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

