Calcitonin and Amylin Receptor Peptide Interaction Mechanisms: INSIGHTS INTO PEPTIDE-BINDING MODES AND ALLOSTERIC MODULATION OF THE CALCITONIN RECEPTOR BY RECEPTOR ACTIVITY-MODIFYING PROTEINS
- PMID: 26895962
- PMCID: PMC4861438
- DOI: 10.1074/jbc.M115.713628
Calcitonin and Amylin Receptor Peptide Interaction Mechanisms: INSIGHTS INTO PEPTIDE-BINDING MODES AND ALLOSTERIC MODULATION OF THE CALCITONIN RECEPTOR BY RECEPTOR ACTIVITY-MODIFYING PROTEINS
Erratum in
-
Calcitonin and amylin receptor peptide interaction mechanisms. INSIGHTS INTO PEPTIDE-BINDING MODES AND ALLOSTERIC MODULATION OF THE CALCITONIN RECEPTOR BY RECEPTOR ACTIVITY-MODIFYING PROTEINS.J Biol Chem. 2016 Jul 29;291(31):16416. doi: 10.1074/jbc.A115.713628. J Biol Chem. 2016. PMID: 27474777 Free PMC article. No abstract available.
Abstract
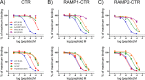
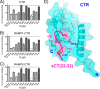
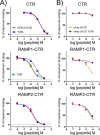
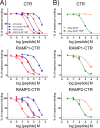
Receptor activity-modifying proteins (RAMP1-3) determine the selectivity of the class B G protein-coupled calcitonin receptor (CTR) and the CTR-like receptor (CLR) for calcitonin (CT), amylin (Amy), calcitonin gene-related peptide (CGRP), and adrenomedullin (AM) peptides. RAMP1/2 alter CLR selectivity for CGRP/AM in part by RAMP1 Trp-84 or RAMP2 Glu-101 contacting the distinct CGRP/AM C-terminal residues. It is unclear whether RAMPs use a similar mechanism to modulate CTR affinity for CT and Amy, analogs of which are therapeutics for bone disorders and diabetes, respectively. Here, we reproduced the peptide selectivity of intact CTR, AMY1 (CTR·RAMP1), and AMY2 (CTR·RAMP2) receptors using purified CTR extracellular domain (ECD) and tethered RAMP1- and RAMP2-CTR ECD fusion proteins and antagonist peptides. All three proteins bound salmon calcitonin (sCT). Tethering RAMPs to CTR enhanced binding of rAmy, CGRP, and the AMY antagonist AC413. Peptide alanine-scanning mutagenesis and modeling of receptor-bound sCT and AC413 supported a shared non-helical CGRP-like conformation for their TN(T/V)G motif prior to the C terminus. After this motif, the peptides diverged; the sCT C-terminal Pro was crucial for receptor binding, whereas the AC413/rAmy C-terminal Tyr had little or no influence on binding. Accordingly, mutant RAMP1 W84A- and RAMP2 E101A-CTR ECD retained AC413/rAmy binding. ECD binding and cell-based signaling assays with antagonist sCT/AC413/rAmy variants with C-terminal residue swaps indicated that the C-terminal sCT/rAmy residue identity affects affinity more than selectivity. rAmy(8-37) Y37P exhibited enhanced antagonism of AMY1 while retaining selectivity. These results reveal unexpected differences in how RAMPs determine CTR and CLR peptide selectivity and support the hypothesis that RAMPs allosterically modulate CTR peptide affinity.
Keywords: G protein-coupled receptor (GPCR); RAMP; allosteric regulation; cell surface receptor; glycoprotein; mutagenesis; peptide hormone; structure-function.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- McLatchie L. M., Fraser N. J., Main M. J., Wise A., Brown J., Thompson N., Solari R., Lee M. G., and Foord S. M. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339 - PubMed
-
- Hay D. L., Poyner D. R., and Sexton P. M. (2006) GPCR modulation by RAMPs. Pharmacol. Ther. 109, 173–197 - PubMed
-
- Poyner D. R., Sexton P. M., Marshall I., Smith D. M., Quirion R., Born W., Muff R., Fischer J. A., and Foord S. M. (2002) International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 54, 233–246 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

