Glia Maturation Factor-γ Regulates Monocyte Migration through Modulation of β1-Integrin
- PMID: 26895964
- PMCID: PMC4861427
- DOI: 10.1074/jbc.M115.674200
Glia Maturation Factor-γ Regulates Monocyte Migration through Modulation of β1-Integrin
Abstract
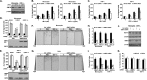
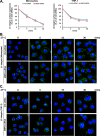
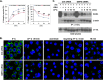
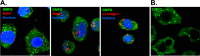
Monocyte migration requires the dynamic redistribution of integrins through a regulated endo-exocytosis cycle, but the complex molecular mechanisms underlying this process have not been fully elucidated. Glia maturation factor-γ (GMFG), a novel regulator of the Arp2/3 complex, has been shown to regulate directional migration of neutrophils and T-lymphocytes. In this study, we explored the important role of GMFG in monocyte chemotaxis, adhesion, and β1-integrin turnover. We found that knockdown of GMFG in monocytes resulted in impaired chemotactic migration toward formyl-Met-Leu-Phe (fMLP) and stromal cell-derived factor 1α (SDF-1α) as well as decreased α5β1-integrin-mediated chemoattractant-stimulated adhesion. These GMFG knockdown impaired effects could be reversed by cotransfection of GFP-tagged full-length GMFG. GMFG knockdown cells reduced the cell surface and total protein levels of α5β1-integrin and increased its degradation. Importantly, we demonstrate that GMFG mediates the ubiquitination of β1-integrin through knockdown or overexpression of GMFG. Moreover, GMFG knockdown retarded the efficient recycling of β1-integrin back to the plasma membrane following normal endocytosis of α5β1-integrin, suggesting that the involvement of GMFG in maintaining α5β1-integrin stability may occur in part by preventing ubiquitin-mediated degradation and promoting β1-integrin recycling. Furthermore, we observed that GMFG interacted with syntaxin 4 (STX4) and syntaxin-binding protein 4 (STXBP4); however, only knockdown of STXBP4, but not STX4, reduced monocyte migration and decreased β1-integrin cell surface expression. Knockdown of STXBP4 also substantially inhibited β1-integrin recycling in human monocytes. These results indicate that the effects of GMFG on monocyte migration and adhesion probably occur through preventing ubiquitin-mediated proteasome degradation of α5β1-integrin and facilitating effective β1-integrin recycling back to the plasma membrane.
Keywords: Arp2/3 complex; adhesion; cell migration; endocytosis; glia maturation factor-gamma (GMFG); integrin; ubiquitylation (ubiquitination).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
Glia maturation factor gamma regulates the migration and adherence of human T lymphocytes.BMC Immunol. 2012 Apr 18;13:21. doi: 10.1186/1471-2172-13-21. BMC Immunol. 2012. PMID: 22510515 Free PMC article.
-
Endothelial cell migration on fibronectin is regulated by syntaxin 6-mediated alpha5beta1 integrin recycling.J Biol Chem. 2011 Oct 21;286(42):36749-61. doi: 10.1074/jbc.M111.260828. Epub 2011 Aug 31. J Biol Chem. 2011. PMID: 21880737 Free PMC article.
-
Glia maturation factor-γ regulates amyloid-β42 phagocytosis through scavenger receptor class A type I in murine macrophages.J Leukoc Biol. 2024 Dec 31;117(1):qiae197. doi: 10.1093/jleuko/qiae197. J Leukoc Biol. 2024. PMID: 39243388 Free PMC article.
-
[Review of the research of glia maturation factor and cloning of human and rat glia maturation factor-gamma (GMFG) cDNA].Nihon Shinkei Seishin Yakurigaku Zasshi. 2001 Feb;21(1):15-20. Nihon Shinkei Seishin Yakurigaku Zasshi. 2001. PMID: 11400321 Review. Japanese.
-
Mechanisms of integrin activation and trafficking.Curr Opin Cell Biol. 2011 Oct;23(5):607-14. doi: 10.1016/j.ceb.2011.08.005. Epub 2011 Sep 14. Curr Opin Cell Biol. 2011. PMID: 21924601 Review.
Cited by
-
Heme induces significant neutrophil adhesion in vitro via an NFκB and reactive oxygen species-dependent pathway.Mol Cell Biochem. 2021 Nov;476(11):3963-3974. doi: 10.1007/s11010-021-04210-5. Epub 2021 Jun 30. Mol Cell Biochem. 2021. PMID: 34191232
-
The Actin-Disassembly Protein Glia Maturation Factor γ Enhances Actin Remodeling and B Cell Antigen Receptor Signaling at the Immune Synapse.Front Cell Dev Biol. 2021 Jul 9;9:647063. doi: 10.3389/fcell.2021.647063. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34336818 Free PMC article.
-
Glia Maturation Factor Dependent Inhibition of Mitochondrial PGC-1α Triggers Oxidative Stress-Mediated Apoptosis in N27 Rat Dopaminergic Neuronal Cells.Mol Neurobiol. 2018 Sep;55(9):7132-7152. doi: 10.1007/s12035-018-0882-6. Epub 2018 Jan 30. Mol Neurobiol. 2018. PMID: 29383690 Free PMC article.
-
Synergy in Disruption of Mitochondrial Dynamics by Aβ (1-42) and Glia Maturation Factor (GMF) in SH-SY5Y Cells Is Mediated Through Alterations in Fission and Fusion Proteins.Mol Neurobiol. 2019 Oct;56(10):6964-6975. doi: 10.1007/s12035-019-1544-z. Epub 2019 Apr 4. Mol Neurobiol. 2019. PMID: 30949973 Free PMC article.
-
Hypoxic Upregulation of IER2 Increases Paracrine GMFG Signaling of Endoplasmic Reticulum Stress-CAF to Promote Chordoma Progression via Targeting ITGB1.Adv Sci (Weinh). 2024 Oct;11(40):e2405421. doi: 10.1002/advs.202405421. Epub 2024 Aug 29. Adv Sci (Weinh). 2024. PMID: 39207055 Free PMC article.
References
-
- Ley K., Laudanna C., Cybulsky M. I., and Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 - PubMed
-
- Wong C. H., Heit B., and Kubes P. (2010) Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc. Res. 86, 183–191 - PubMed
-
- Herter J., and Zarbock A. (2013) Integrin regulation during leukocyte recruitment. J. Immunol. 190, 4451–4457 - PubMed
-
- Steeber D. A., and Tedder T. F. (2000) Adhesion molecule cascades direct lymphocyte recirculation and leukocyte migration during inflammation. Immunol. Res. 22, 299–317 - PubMed
-
- Chuluyan H. E., Schall T. J., Yoshimura T., and Issekutz A. C. (1995) IL-1 activation of endothelium supports VLA-4 (CD49d/CD29)-mediated monocyte transendothelial migration to C5a, MIP-1α, RANTES, and PAF but inhibits migration to MCP-1: a regulatory role for endothelium-derived MCP-1. J. Leukoc. Biol. 58, 71–79 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

