Lactococcus lactis LMG2081 Produces Two Bacteriocins, a Nonlantibiotic and a Novel Lantibiotic
- PMID: 26896142
- PMCID: PMC4959506
- DOI: 10.1128/AEM.03988-15
Lactococcus lactis LMG2081 Produces Two Bacteriocins, a Nonlantibiotic and a Novel Lantibiotic
Abstract
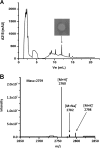
Bacteriocin producers normally possess dedicated immunity systems to protect themselves from their own bacteriocins.Lactococcus lactis strains LMG2081 and BGBM50 are known as lactococcin G producers. However, BGBM50 was sensitive to LMG2081, which indicated that LMG2081 might produce additional bacteriocins that are not present in BGBM50. Therefore, whole-genome sequencing of the two strains was performed, and a lantibiotic operon (called lctLMG) was identified in LMG2081 but not in BGBM50. The lctLMG operon contains six open reading frames; the first three genes,lmgA ,lmgM, and lmgT, are involved in the biosynthesis and export of bacteriocin, while the other three genes,lmgF,lmgE, and lmgG, are involved in lantibiotic immunity. Mutational analysis confirmed that the lctLMG operon is responsible for the additional antimicrobial activity. Specifically, site-directed mutation within this operon rendered LMG2081 inactive toward BGBM50. Subsequent purification and electrospray ionization-time of flight mass spectrometric analysis confirmed that the lantibiotic bacteriocin called lacticin LMG is exported as a 25-amino-acid peptide. Lacticin LMG is highly similar to the lacticin 481 group. It is interesting that a bacteriocin producer produces two different classes of bacteriocins, whose operons are located in the chromosome and a plasmid.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

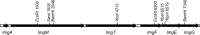


Similar articles
-
Characterization of the lacticin 481 operon: the Lactococcus lactis genes lctF, lctE, and lctG encode a putative ABC transporter involved in bacteriocin immunity.Appl Environ Microbiol. 1997 Nov;63(11):4252-60. doi: 10.1128/aem.63.11.4252-4260.1997. Appl Environ Microbiol. 1997. PMID: 9361411 Free PMC article.
-
Identification of Lactococcus-Specific Bacteriocins Produced by Lactococcal Isolates, and the Discovery of a Novel Bacteriocin, Lactococcin Z.Probiotics Antimicrob Proteins. 2015 Sep;7(3):222-31. doi: 10.1007/s12602-015-9196-4. Probiotics Antimicrob Proteins. 2015. PMID: 26093857
-
Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from lactococcus lactis subsp. lactis.Appl Environ Microbiol. 1994 May;60(5):1652-7. doi: 10.1128/aem.60.5.1652-1657.1994. Appl Environ Microbiol. 1994. PMID: 8017945 Free PMC article.
-
Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene.J Bacteriol. 1991 Jun;173(12):3879-87. doi: 10.1128/jb.173.12.3879-3887.1991. J Bacteriol. 1991. PMID: 1904860 Free PMC article.
-
Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria.Microbiology (Reading). 2011 Dec;157(Pt 12):3256-3267. doi: 10.1099/mic.0.052571-0. Epub 2011 Oct 6. Microbiology (Reading). 2011. PMID: 21980118 Review.
Cited by
-
Exploring Beneficial Properties of the Bacteriocinogenic Enterococcus faecium ST10Bz Strain Isolated from Boza, a Bulgarian Cereal-Based Beverage.Microorganisms. 2020 Sep 25;8(10):1474. doi: 10.3390/microorganisms8101474. Microorganisms. 2020. PMID: 32992853 Free PMC article.
-
Lactolisterin BU, a Novel Class II Broad-Spectrum Bacteriocin from Lactococcus lactis subsp. lactis bv. diacetylactis BGBU1-4.Appl Environ Microbiol. 2017 Oct 17;83(21):e01519-17. doi: 10.1128/AEM.01519-17. Print 2017 Nov 1. Appl Environ Microbiol. 2017. PMID: 28842543 Free PMC article.
-
A Multibacteriocin Cheese Starter System, Comprising Nisin and Lacticin 3147 in Lactococcus lactis, in Combination with Plantaricin from Lactobacillus plantarum.Appl Environ Microbiol. 2017 Jun 30;83(14):e00799-17. doi: 10.1128/AEM.00799-17. Print 2017 Jul 15. Appl Environ Microbiol. 2017. PMID: 28476774 Free PMC article.
-
Pseudomonas koreensis Recovered From Raw Yak Milk Synthesizes a β-Carboline Derivative With Antimicrobial Properties.Front Microbiol. 2019 Jul 29;10:1728. doi: 10.3389/fmicb.2019.01728. eCollection 2019. Front Microbiol. 2019. PMID: 31417521 Free PMC article.
-
Assessment of Synthesis Machinery of Two Antimicrobial Peptides from Paenibacillus alvei NP75.Probiotics Antimicrob Proteins. 2020 Mar;12(1):39-47. doi: 10.1007/s12602-019-09541-w. Probiotics Antimicrob Proteins. 2020. PMID: 31001787
References
-
- Nes IF. 2011. History, current knowledge, and future directions on bacteriocin research in lactic acid bacteria, p 3–12. In Drider D, Rebuffat S (ed), Prokaryotic antimicrobial peptides: from genes to applications. Springer Science & Business Media, New York, NY.
-
- Cotter PD, Ross RP, Hill C. 2013. Bacteriocins: a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105. - PubMed
-
- Jung G. 1991. Nisin and novel lantibiotics, p 1–34. In Jung G, Sahl H-G (ed), Nisin and novel lantibiotics. ESCOM, Leiden, Netherlands.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

