Tamoxifen Administration Immediately or 24 Hours after Spinal Cord Injury Improves Locomotor Recovery and Reduces Secondary Damage in Female Rats
- PMID: 26896212
- PMCID: PMC5035917
- DOI: 10.1089/neu.2015.4111
Tamoxifen Administration Immediately or 24 Hours after Spinal Cord Injury Improves Locomotor Recovery and Reduces Secondary Damage in Female Rats
Abstract
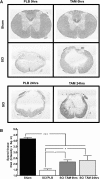
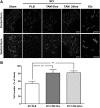
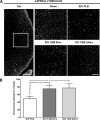
Spinal cord injury (SCI) is a condition with no available cure. The initial physical impact triggers a cascade of molecular and cellular events that generate a nonpermissive environment for cell survival and axonal regeneration. Spinal cord injured patients often arrive at the clinic hours after the initial insult. This indicates the need to study and develop treatments with a long therapeutic window of action and multiactive properties, which target the complex set of events that arise after the initial trauma. We provide evidence that tamoxifen (TAM), a drug approved by the Food and Drug Administration, exerts neuroprotective effects in an animal model when applied up-to 24 h after SCI. We hypothesized that continuous TAM administration will improve functional locomotor recovery by favoring myelin preservation and reducing secondary damage after SCI. Adult female Sprague-Dawley rats (∼230 g) received a moderate contusion to the thoracic (T9-T10) spinal cord, using the MASCIS impactor device. To determine the therapeutic window available for TAM treatment, rats were implanted with TAM pellets (15 mg) immediately or 24 h after SCI. Locomotor function (Basso, Beattie, Bresnahan open field test, grid walk, and beam crossing tests) was assessed weekly for 35 days post-injury. TAM-treated rats showed significant functional locomotor recovery and improved fine movements when treated immediately or 24 h after SCI. Further, TAM increased white matter preservation and reduced secondary damage caused by astrogliosis, axonal degeneration, and cell death after trauma. These results provide evidence for TAM as a potential therapeutic agent to treat SCI up to 24 h after the trauma.
Keywords: astrogliosis; neuroprotection; selective estrogen receptor modulator (SERM); spare tissue; therapeutic drug.
Conflict of interest statement
Author Disclosure Statement No competing financial interests exist.
Figures


References
-
- Rabchevsky A.G., Patel S.P., and Springer J.E. (2011). Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol. Ther. 132, 15–29 - PubMed
-
- McKerracher L., and Higuchi H. (2006). Targeting Rho to stimulate repair after spinal cord injury. J. Neurotrauma 23, 309–17 - PubMed
-
- Hulsebosch C.E. (2002). Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol. Educ. 26, 238–255 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

