Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen
- PMID: 26896232
- PMCID: PMC4852987
- DOI: 10.1016/j.jaci.2015.12.1322
Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen
Abstract
Background: The influence of early exposure to allergenic foods on the subsequent development of food allergy remains uncertain.
Objective: We sought to determine the feasibility of the early introduction of multiple allergenic foods to exclusively breast-fed infants from 3 months of age and the effect on breastfeeding performance.
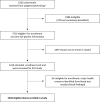
Methods: We performed a randomized controlled trial. The early introduction group (EIG) continued breastfeeding with sequential introduction of 6 allergenic foods: cow's milk, peanut, hard-boiled hen's egg, sesame, whitefish (cod), and wheat; the standard introduction group followed the UK infant feeding recommendations of exclusive breastfeeding for around 6 months with no introduction of allergenic foods before 6 months of age.
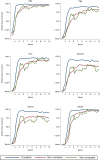
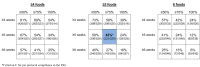
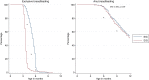
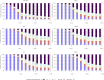
Results: One thousand three hundred three infants were enrolled. By 5 months of age, the median frequency of consumption of all 6 foods was 2 to 3 times per week for every food in the EIG and no consumption for every food in the standard introduction group (P < .001 for every comparison). By 6 months of age, nonintroduction of the allergenic foods in the EIG was less than 5% for each of the 6 foods. Achievement of the stringent per-protocol consumption target for the EIG proved more difficult (42% of evaluable EIG participants). Breastfeeding rates in both groups significantly exceeded UK government data for equivalent mothers (P < .001 at 6 and at 9 months of age).
Conclusion: Early introduction, before 6 months of age, of at least some amount of multiple allergenic foods appears achievable and did not affect breastfeeding. This has important implications for the evaluation of food allergy prevention strategies.
Keywords: Food allergy; allergens; breastfeeding; diet; infancy.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



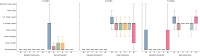
References
-
- Nwaru B.I., Hickstein L., Panesar S.S., Roberts G., Muraro A., Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69:992–1007. - PubMed
-
- Grundy J., Matthews S., Bateman B., Dean T., Arshad S.H. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110:784–789. - PubMed
-
- World Health Organization . World Health Organization; Geneva: 2003. Global strategy for infant and young child feeding.
-
- Department of Health. Infant feeding recommendation. 2003. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http:/www.dh.go.... Accessed January 15, 2016.
-
- World Health Organization . World Health Organization; Geneva: 2011. Exclusive breastfeeding for six months best for babies everywhere.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

