Bap1 Is a Bona Fide Tumor Suppressor: Genetic Evidence from Mouse Models Carrying Heterozygous Germline Bap1 Mutations
- PMID: 26896281
- PMCID: PMC4873414
- DOI: 10.1158/0008-5472.CAN-15-3371
Bap1 Is a Bona Fide Tumor Suppressor: Genetic Evidence from Mouse Models Carrying Heterozygous Germline Bap1 Mutations
Abstract
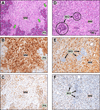
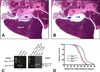
Individuals harboring inherited heterozygous germline mutations in BAP1 are predisposed to a range of benign and malignant tumor types, including malignant mesothelioma, melanoma, and kidney carcinoma. However, evidence to support a tumor-suppressive role for BAP1 in cancer remains contradictory. To test experimentally whether BAP1 behaves as a tumor suppressor, we monitored spontaneous tumor development in three different mouse models with germline heterozygous mutations in Bap1, including two models in which the knock-in mutations are identical to those reported in human BAP1 cancer syndrome families. We observed spontaneous malignant tumors in 54 of 93 Bap1-mutant mice (58%) versus 4 of 43 (9%) wild-type littermates. All three Bap1-mutant models exhibited a high incidence and similar spectrum of neoplasms, including ovarian sex cord stromal tumors, lung and mammary carcinomas, and spindle cell tumors. Notably, we also observed malignant mesotheliomas in two Bap1-mutant mice, but not in any wild-type animals. We further confirmed that the remaining wild-type Bap1 allele was lost in both spontaneous ovarian tumors and mesotheliomas, resulting in the loss of Bap1 expression. Additional studies revealed that asbestos exposure induced a highly significant increase in the incidence of aggressive mesotheliomas in the two mouse models carrying clinically relevant Bap1 mutations compared with asbestos-exposed wild-type littermates. Collectively, these findings provide genetic evidence that Bap1 is a bona fide tumor suppressor gene and offer key insights into the contribution of carcinogen exposure to enhanced cancer susceptibility. Cancer Res; 76(9); 2836-44. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
All other authors declare no potential conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

