Structural studies reveal an important role for the pleiotrophin C-terminus in mediating interactions with chondroitin sulfate
- PMID: 26896299
- PMCID: PMC6217956
- DOI: 10.1111/febs.13686
Structural studies reveal an important role for the pleiotrophin C-terminus in mediating interactions with chondroitin sulfate
Abstract
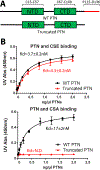
Pleiotrophin (PTN) is a potent glycosaminoglycan-binding cytokine that is important in neural development, angiogenesis and tissue regeneration. Much of its activity is attributed to its interactions with the chondroitin sulfate (CS) proteoglycan, receptor type protein tyrosine phosphatase ζ (PTPRZ). However, there is little high resolution structural information on the interactions between PTN and CS, nor is it clear why the C-terminal tail of PTN is necessary for signaling through PTPRZ, even though it does not contribute to heparin binding. We determined the first structure of PTN and analyzed its interactions with CS. Our structure shows that PTN possesses large basic surfaces on both of its structured domains and also that residues in the hinge segment connecting the domains have significant contacts with the C-terminal domain. Our analysis of PTN-CS interactions showed that the C-terminal tail of PTN is essential for maintaining stable interactions with chondroitin sulfate A, the type of CS commonly found on PTPRZ. These results offer the first possible explanation of why truncated PTN missing the C-terminal tail is unable to signal through PTPRZ. NMR analysis of the interactions of PTN with CS revealed that the C-terminal domain and hinge of PTN make up the major CS-binding site in PTN, and that removal of the C-terminal tail weakened the affinity of the site for CSA but not for other high sulfation density CS.
Database: Coordinates of the ensemble of ten PTN structures have been deposited in RCSB under accession number 2n6f. Chemical shifts assignments and structural constraints have been deposited in BMRB under accession number 25762.
Keywords: NMR; cytokine; glycosaminoglycan-binding protein; glycosaminolgycan.
© 2016 Federation of European Biochemical Societies.
Figures




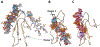
Similar articles
-
Pleiotrophin: Activity and mechanism.Adv Clin Chem. 2020;98:51-89. doi: 10.1016/bs.acc.2020.02.003. Epub 2020 Mar 12. Adv Clin Chem. 2020. PMID: 32564788 Free PMC article. Review.
-
Role of Chondroitin Sulfate (CS) Modification in the Regulation of Protein-tyrosine Phosphatase Receptor Type Z (PTPRZ) Activity: PLEIOTROPHIN-PTPRZ-A SIGNALING IS INVOLVED IN OLIGODENDROCYTE DIFFERENTIATION.J Biol Chem. 2016 Aug 26;291(35):18117-28. doi: 10.1074/jbc.M116.742536. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445335 Free PMC article.
-
Demonstration of the pleiotrophin-binding oligosaccharide sequences isolated from chondroitin sulfate/dermatan sulfate hybrid chains of embryonic pig brains.J Biol Chem. 2005 Oct 21;280(42):35318-28. doi: 10.1074/jbc.M507304200. Epub 2005 Aug 23. J Biol Chem. 2005. PMID: 16120610
-
Heterogeneity of the chondroitin sulfate portion of phosphacan/6B4 proteoglycan regulates its binding affinity for pleiotrophin/heparin binding growth-associated molecule.J Biol Chem. 2003 Sep 12;278(37):35805-11. doi: 10.1074/jbc.M305530200. Epub 2003 Jul 2. J Biol Chem. 2003. PMID: 12840014
-
Chondroitin sulfates and their binding molecules in the central nervous system.Glycoconj J. 2017 Jun;34(3):363-376. doi: 10.1007/s10719-017-9761-z. Epub 2017 Jan 18. Glycoconj J. 2017. PMID: 28101734 Free PMC article. Review.
Cited by
-
Pleiotrophin: Activity and mechanism.Adv Clin Chem. 2020;98:51-89. doi: 10.1016/bs.acc.2020.02.003. Epub 2020 Mar 12. Adv Clin Chem. 2020. PMID: 32564788 Free PMC article. Review.
-
Pleiotrophin, a multifunctional cytokine and growth factor, induces leukocyte responses through the integrin Mac-1.J Biol Chem. 2017 Nov 17;292(46):18848-18861. doi: 10.1074/jbc.M116.773713. Epub 2017 Sep 22. J Biol Chem. 2017. PMID: 28939773 Free PMC article.
-
αMI-domain of Integrin Mac-1 Binds the Cytokine Pleiotrophin Using Multiple Mechanisms.bioRxiv [Preprint]. 2024 Feb 2:2024.02.01.578455. doi: 10.1101/2024.02.01.578455. bioRxiv. 2024. Update in: Structure. 2024 Aug 8;32(8):1184-1196.e4. doi: 10.1016/j.str.2024.04.013. PMID: 38352421 Free PMC article. Updated. Preprint.
-
Structural Characterization of the Interaction between the αMI-Domain of the Integrin Mac-1 (αMβ2) and the Cytokine Pleiotrophin.Biochemistry. 2021 Jan 26;60(3):182-193. doi: 10.1021/acs.biochem.0c00700. Epub 2021 Jan 11. Biochemistry. 2021. PMID: 33427449 Free PMC article.
-
The carboxy-terminus, a key regulator of protein function.Crit Rev Biochem Mol Biol. 2019 Apr;54(2):85-102. doi: 10.1080/10409238.2019.1586828. Epub 2019 May 20. Crit Rev Biochem Mol Biol. 2019. PMID: 31106589 Free PMC article. Review.
References
-
- Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrandt J & Deuel TF (1990) Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity, Science. 250, 1690–4. - PubMed
-
- Silos-Santiago I, Yeh HJ, Gurrieri MA, Guillerman RP, Li YS, Wolf J, Snider W & Deuel TF (1996) Localization of pleiotrophin and its mRNA in subpopulations of neurons and their corresponding axonal tracts suggests important roles in neural-glial interactions during development and in maturity, J Neurobiol. 31, 283–96. - PubMed
-
- Himburg HA, Harris JR, Ito T, Daher P, Russell JL, Quarmyne M, Doan PL, Helms K, Nakamura M, Fixsen E, Herradon G, Reya T, Chao NJ, Harroch S & Chute JP (2012) Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche, Cell Rep. 2, 964–75. - PMC - PubMed
-
- Hatziapostolou M, Delbe J, Katsoris P, Polytarchou C, Courty J & Papadimitriou E (2005) Heparin affin regulatory peptide is a key player in prostate cancer cell growth and angiogenicity, Prostate. 65, 151–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

