Influenza B vaccine lineage selection--an optimized trivalent vaccine
- PMID: 26896685
- PMCID: PMC4793086
- DOI: 10.1016/j.vaccine.2016.01.042
Influenza B vaccine lineage selection--an optimized trivalent vaccine
Abstract
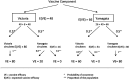
Epidemics of seasonal influenza viruses cause considerable morbidity and mortality each year. Various types and subtypes of influenza circulate in humans and evolve continuously such that individuals at risk of serious complications need to be vaccinated annually to keep protection up to date with circulating viruses. The influenza vaccine in most parts of the world is a trivalent vaccine, including an antigenically representative virus of recently circulating influenza A/H3N2, A/H1N1, and influenza B viruses. However, since the 1970s influenza B has split into two antigenically distinct lineages, only one of which is represented in the annual trivalent vaccine at any time. We describe a lineage selection strategy that optimizes protection against influenza B using the standard trivalent vaccine as a potentially cost effective alternative to quadrivalent vaccines.
Keywords: Decision tree; Hedging; Influenza B; Quadrivalent; Trivalent vaccine; Vaccine strain selection.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- WHO . WHO; 2003. Fact sheet no. 211 influenza. Available from: 〈http://www.who.int/mediacentre/factsheets/2003/fs211/en/〉.
-
- Grohskopf L.A., Olsen S.J., Sokolow L.Z., Bresee J.S., Cox N.J., Broder K.R. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691–697. - PMC - PubMed
-
- Klimov A.I., Garten R., Russell C., Barr I.G., Besselaar T.G., Daniels R. WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere Influenza Vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from February to September 2011. Vaccine. 2012;30(45):6461–6471. - PMC - PubMed
-
- Barr I.G., Russell C., Besselaar T.G., Cox N.J., Daniels R.S., Donis R. WHO recommendations for the viruses used in the 2013–2014 Northern Hemisphere influenza vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from October 2012 to January 2013. Vaccine. 2014;32(37):4713–4725. - PubMed
-
- Barr I.G., McCauley J., Cox N., Daniels R., Engelhardt O.G., Fukuda K. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 northern hemisphere season. Vaccine. 2010;28(5):1156–1167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

