Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study
- PMID: 26897105
- PMCID: PMC4909992
- DOI: 10.1016/S1473-3099(16)00054-2
Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study
Abstract
Background: Little information is available about the effect of pneumococcal conjugate vaccines (PCVs) in low-income countries. We measured the effect of these vaccines on invasive pneumococcal disease in The Gambia where the 7-valent vaccine (PCV7) was introduced in August, 2009, followed by the 13-valent vaccine (PCV13) in May, 2011.
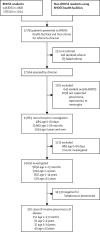
Methods: We conducted population-based surveillance for invasive pneumococcal disease in individuals aged 2 months and older who were residents of the Basse Health and Demographic Surveillance System (BHDSS) in the Upper River Region, The Gambia, using standardised criteria to identify and investigate patients. Surveillance was done between May, 2008, and December, 2014. We compared the incidence of invasive pneumococcal disease between baseline (May 12, 2008-May 11, 2010) and after the introduction of PCV13 (Jan 1, 2013-Dec 31, 2014), adjusting for changes in case ascertainment over time.
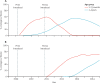
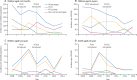
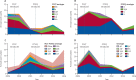
Findings: We investigated 14 650 patients, in whom we identified 320 cases of invasive pneumococcal disease. Compared with baseline, after the introduction of the PCV programme, the incidence of invasive pneumococcal disease decreased by 55% (95% CI 30-71) in the 2-23 months age group, from 253 to 113 per 100 000 population. This decrease was due to an 82% (95% CI 64-91) reduction in serotypes covered by the PCV13 vaccine. In the 2-4 years age group, the incidence of invasive pneumococcal disease decreased by 56% (95% CI 25-75), from 113 to 49 cases per 100 000, with a 68% (95% CI 39-83) reduction in PCV13 serotypes. The incidence of non-PCV13 serotypes in children aged 2-59 months increased by 47% (-21 to 275) from 28 to 41 per 100 000, with a broad range of serotypes. The incidence of non-pneumococcal bacteraemia varied little over time.
Interpretation: The Gambian PCV programme reduced the incidence of invasive pneumococcal disease in children aged 2-59 months by around 55%. Further surveillance is needed to ascertain the maximum effect of the vaccine in the 2-4 years and older age groups, and to monitor serotype replacement. Low-income and middle-income countries that introduce PCV13 can expect substantial reductions in invasive pneumococcal disease.
Funding: GAVI's Pneumococcal vaccines Accelerated Development and Introduction Plan (PneumoADIP), Bill & Melinda Gates Foundation, and the UK Medical Research Council.
Copyright © 2016 Mackenzie et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Long-term surveillance of the effect of PCV13: the future challenge in Africa.Lancet Infect Dis. 2016 Jun;16(6):627-629. doi: 10.1016/S1473-3099(16)00059-1. Epub 2016 Feb 18. Lancet Infect Dis. 2016. PMID: 26897107 No abstract available.
References
-
- World Health Organization Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008 (updated Dec 1, 2013) http://www.who.int/immunization/monitoring_surveillance/burden/estimates... (accessed Oct 15, 2015).
-
- Pilishvili T, Lexau C, Farley MM. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. - PubMed
-
- de Kluyver R. Invasive pneumococcal disease surveillance Australia, 1 April to 30 June 2014. Commun Dis Intell Q Rep. 2014;38:E266–E270. - PubMed
-
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–543. - PubMed
-
- Vaccine Information Management System Report on Global Introduction (updated September 21, 2015) http://www.jhsph.edu/research/centers-and-institutes/ivac/vims/ (accessed Oct 1, 2015).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

