Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling
- PMID: 26898330
- PMCID: PMC4785881
- DOI: 10.1016/j.cell.2016.01.032
Axon Degeneration Gated by Retrograde Activation of Somatic Pro-apoptotic Signaling
Abstract
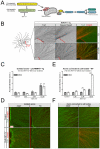
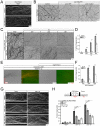
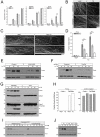
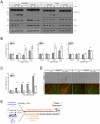
During development, sensory axons compete for limiting neurotrophic support, and local neurotrophin insufficiency triggers caspase-dependent axon degeneration. The signaling driving axon degeneration upon local deprivation is proposed to reside within axons. Our results instead support a model in which, despite the apoptotic machinery being present in axons, the cell body is an active participant in gating axonal caspase activation and axon degeneration. Loss of trophic support in axons initiates retrograde activation of a somatic pro-apoptotic pathway, which, in turn, is required for distal axon degeneration via an anterograde pro-degenerative factor. At a molecular level, the cell body is the convergence point of two signaling pathways whose integrated action drives upregulation of pro-apoptotic Puma, which, unexpectedly, is confined to the cell body. Puma then overcomes inhibition by pro-survival Bcl-xL and Bcl-w and initiates the anterograde pro-degenerative program, highlighting the role of the cell body as an arbiter of large-scale axon removal.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Ground Control to Major Tom: The Cell Body Signals Axon Degeneration.Cell. 2016 Feb 25;164(5):842-4. doi: 10.1016/j.cell.2016.02.013. Cell. 2016. PMID: 26919423
References
-
- Akhter R, Sanphui P, Das H, Saha P, Biswas SC. The regulation of p53 up-regulated modulator of apoptosis by JNK/c-Jun pathway in beta-amyloid-induced neuron death. Journal of neurochemistry. 2015;134:1091–1103. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

