Microfluidic model of the platelet-generating organ: beyond bone marrow biomimetics
- PMID: 26898346
- PMCID: PMC4761988
- DOI: 10.1038/srep21700
Microfluidic model of the platelet-generating organ: beyond bone marrow biomimetics
Abstract
We present a new, rapid method for producing blood platelets in vitro from cultured megakaryocytes based on a microfluidic device. This device consists in a wide array of VWF-coated micropillars. Such pillars act as anchors on megakaryocytes, allowing them to remain trapped in the device and subjected to hydrodynamic shear. The combined effect of anchoring and shear induces the elongation of megakaryocytes and finally their rupture into platelets and proplatelets. This process was observed with megakaryocytes from different origins and found to be robust. This original bioreactor design allows to process megakaryocytes at high throughput (millions per hour). Since platelets are produced in such a large amount, their extensive biological characterisation is possible and shows that platelets produced in this bioreactor are functional.
Conflict of interest statement
PlatOD, ESPCI INNOV, and the authors (A.B., A.L.G., A.M., S.P.-C., D.B. and M.R.) have a patent related to this work: “Fluidic device for producing platelets” (International Publication Number WO2015/075030 A1).
Figures
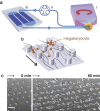
 wide,
wide,  deep and
deep and  long. The dark blue region corresponds to the pillar forests that cover the straight part of the channels, while the U-turns are devoid of obstacles. (b) Pillars are arranged on a tilted hexagonal lattice. The angle between the main axis of the channel and that of the lattice is fixed to 10° in order to ensure that each cell encounters at least one pillar. The streamlines are illustrated with black lines and MKs with orange spheres. (c) Top view of the micro pillar array before cell perfusion and after 60 minutes perfusion. The capture process of cells at intermediate times will be described in Fig. 2. Scale bar, 100
long. The dark blue region corresponds to the pillar forests that cover the straight part of the channels, while the U-turns are devoid of obstacles. (b) Pillars are arranged on a tilted hexagonal lattice. The angle between the main axis of the channel and that of the lattice is fixed to 10° in order to ensure that each cell encounters at least one pillar. The streamlines are illustrated with black lines and MKs with orange spheres. (c) Top view of the micro pillar array before cell perfusion and after 60 minutes perfusion. The capture process of cells at intermediate times will be described in Fig. 2. Scale bar, 100  m.
m.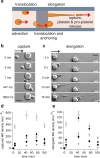
 then translocates around a pillar. In advection it takes 3 ms for the MK to move 3 times its diameter, while 1.8 s are necessary to roll over the same distance by translocation. Scale bar, 20
then translocates around a pillar. In advection it takes 3 ms for the MK to move 3 times its diameter, while 1.8 s are necessary to roll over the same distance by translocation. Scale bar, 20  m. (c) Top view of a MK elongation. The montage starts at −2 s, before the MK is captured by the left pillar. Scale bar, 20
m. (c) Top view of a MK elongation. The montage starts at −2 s, before the MK is captured by the left pillar. Scale bar, 20  m. (d) Surface density of captured MKs as a function of time during a perfusion of 6,7 ml at 200 000 MK/mL suspension through 1 chip, measured at different positions:
m. (d) Surface density of captured MKs as a function of time during a perfusion of 6,7 ml at 200 000 MK/mL suspension through 1 chip, measured at different positions:  mm (
mm ( ),
),  mm
mm  ,
,  mm
mm  ,
,  mm
mm  ,
,  mm (
mm ( ). Counts were performed by two experimentalists and represented as (mean ± SD). (e) Surface density of elongated MKs as a function of time for the same conditions.
). Counts were performed by two experimentalists and represented as (mean ± SD). (e) Surface density of elongated MKs as a function of time for the same conditions.
 ). The origin of time is defined as the moment when the circulating MK is captured by a pillar. The first rupture occurs at t = 27 min, when a portion of string bearing 6 beads detaches from the rest of the cell. Other similar events occur at later times, involving one (t = 29 or 31 min) or several (t = 46 min) beads. Scale bar, 20
). The origin of time is defined as the moment when the circulating MK is captured by a pillar. The first rupture occurs at t = 27 min, when a portion of string bearing 6 beads detaches from the rest of the cell. Other similar events occur at later times, involving one (t = 29 or 31 min) or several (t = 46 min) beads. Scale bar, 20  m. (b) Length of a megakaryocyte (n = 1). Stars (*) indicate fragmentation and numbers indicate different phases of the elongation process: (1) reorganization of proplatelets, (2) anchoring to a 2nd pillar, (3) dissociation from 1st pillar, (4) reorganization of proplatelets, (5) untangling of megakaryocyte segments (6) retraction of the residual chain. The time t = 0 min corresponds to the megakaryocyte anchorage to the first pillar. The sequence of pictures presented in panel (a) corresponds to snapshots taken between times depicted by arrows 3 and 5. The data are extracted from video 3.
m. (b) Length of a megakaryocyte (n = 1). Stars (*) indicate fragmentation and numbers indicate different phases of the elongation process: (1) reorganization of proplatelets, (2) anchoring to a 2nd pillar, (3) dissociation from 1st pillar, (4) reorganization of proplatelets, (5) untangling of megakaryocyte segments (6) retraction of the residual chain. The time t = 0 min corresponds to the megakaryocyte anchorage to the first pillar. The sequence of pictures presented in panel (a) corresponds to snapshots taken between times depicted by arrows 3 and 5. The data are extracted from video 3.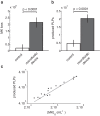
 in the reservoir during a 2 h perfusion (20 mL, 5 chips). (c) Influence of the suspension concentration
in the reservoir during a 2 h perfusion (20 mL, 5 chips). (c) Influence of the suspension concentration  on the number
on the number  of released platelet-like-particles during a 2 h perfusion with a small volume of culture (6.7 mL). The curve can be fitted with a linear law (slope
of released platelet-like-particles during a 2 h perfusion with a small volume of culture (6.7 mL). The curve can be fitted with a linear law (slope  , correlation coefficient
, correlation coefficient  ).
).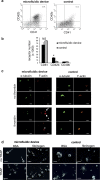
 -tubulin antibody, revealed by a secondary AlexaFluor488 goat anti-mouse antibody and AlexaFluor546 phalloidin for F-actin staining is performed in the absence (top panel) or presence (bottom panel) of thrombin. Circular tubulin staining, characteristic of unactivated platelets is seen in the samples collected at the exit of the fluidic device (top left), whereas larger fragments without circular tubulin staining are recovered in samples collected from the control (top right). Actin stress fibres (small arrow, full arrow indicate a filopod and dotted arrow a lamellipod) characteristic of activated platelets are seen in the samples collected at the exit of the fluidic device (bottom left), whereas larger elements without organised stress fibres staining are recovered in samples collected from the control (bottom right). Platelets are adherent to fibrinogen. Scale bar 5
-tubulin antibody, revealed by a secondary AlexaFluor488 goat anti-mouse antibody and AlexaFluor546 phalloidin for F-actin staining is performed in the absence (top panel) or presence (bottom panel) of thrombin. Circular tubulin staining, characteristic of unactivated platelets is seen in the samples collected at the exit of the fluidic device (top left), whereas larger fragments without circular tubulin staining are recovered in samples collected from the control (top right). Actin stress fibres (small arrow, full arrow indicate a filopod and dotted arrow a lamellipod) characteristic of activated platelets are seen in the samples collected at the exit of the fluidic device (bottom left), whereas larger elements without organised stress fibres staining are recovered in samples collected from the control (bottom right). Platelets are adherent to fibrinogen. Scale bar 5  m. (d) Electron microscopy observations of platelets on BSA or fibrinogen coated surface, upon TRAP activation. Discoid round platelets are indicated with full white arrows and pseudopods with dotted arrows. Scale bar 5
m. (d) Electron microscopy observations of platelets on BSA or fibrinogen coated surface, upon TRAP activation. Discoid round platelets are indicated with full white arrows and pseudopods with dotted arrows. Scale bar 5  m.
m.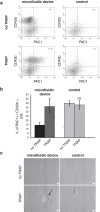
 m.
m.References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

