TPhP exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver
- PMID: 26898711
- PMCID: PMC4761896
- DOI: 10.1038/srep21827
TPhP exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver
Abstract
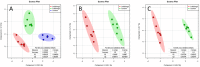
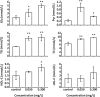
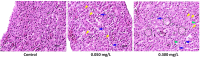
Triphenyl phosphate is a high production volume organophosphate flame retardant that has been detected in multiple environmental media at increasing concentrations. The environmental and health risks of triphenyl phosphate have drawn attention because of the multiplex toxicity of this chemical compound. However, few studies have paid close attention to the impacts of triphenyl phosphate on liver metabolism. We investigated hepatic histopathological, metabolomic and transcriptomic responses of zebrafish after exposure to 0.050 mg/L and 0.300 mg/L triphenyl phosphate for 7 days. Metabolomic analysis revealed significant changes in the contents of glucose, UDP-glucose, lactate, succinate, fumarate, choline, acetylcarnitine, and several fatty acids. Transcriptomic analysis revealed that related pathways, such as the glycosphingolipid biosynthesis, PPAR signaling pathway and fatty acid elongation, were significantly affected. These results suggest that triphenyl phosphate exposure markedly disturbs hepatic carbohydrate and lipid metabolism in zebrafish. Moreover, DNA replication, the cell cycle, and non-homologous end-joining and base excision repair were strongly affected, thus indicating that triphenyl phosphate hinders the DNA damage repair system in zebrafish liver cells. The present study provides a systematic analysis of the triphenyl phosphate-induced toxic effects in zebrafish liver and demonstrates that low concentrations of triphenyl phosphate affect normal metabolism and cell cycle.
Figures


Similar articles
-
Acute exposure to triphenyl phosphate (TPhP) disturbs ocular development and muscular organization in zebrafish larvae.Ecotoxicol Environ Saf. 2019 Sep 15;179:119-126. doi: 10.1016/j.ecoenv.2019.04.056. Epub 2019 Apr 28. Ecotoxicol Environ Saf. 2019. PMID: 31035246
-
Uptake and toxic effects of triphenyl phosphate on freshwater microalgae Chlorella vulgaris and Scenedesmus obliquus: Insights from untargeted metabolomics.Sci Total Environ. 2019 Feb 10;650(Pt 1):1239-1249. doi: 10.1016/j.scitotenv.2018.09.024. Epub 2018 Sep 5. Sci Total Environ. 2019. PMID: 30308812
-
Organophosphorus flame retardant induced hepatotoxicity and brain AChE inhibition on zebrafish (Danio rerio).Neurotoxicol Teratol. 2020 Nov-Dec;82:106919. doi: 10.1016/j.ntt.2020.106919. Epub 2020 Aug 24. Neurotoxicol Teratol. 2020. PMID: 32853706
-
Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis.Chemosphere. 2012 Aug;88(10):1119-53. doi: 10.1016/j.chemosphere.2012.03.067. Epub 2012 Apr 25. Chemosphere. 2012. PMID: 22537891 Review.
-
Metabolism and genotoxicity of the halogenated alkyl compound tris(2,3-dibromopropyl)phosphate.Hum Exp Toxicol. 1994 Dec;13(12):861-5. doi: 10.1177/096032719401301208. Hum Exp Toxicol. 1994. PMID: 7718306 Review.
Cited by
-
Triphenyl Phosphate Alters Methyltransferase Expression and Induces Genome-Wide Aberrant DNA Methylation in Zebrafish Larvae.Chem Res Toxicol. 2024 Sep 16;37(9):1549-1561. doi: 10.1021/acs.chemrestox.4c00223. Epub 2024 Aug 29. Chem Res Toxicol. 2024. PMID: 39205618 Free PMC article.
-
Integration of microRNAome, proteomics and metabolomics to analyze arsenic-induced malignant cell transformation.Oncotarget. 2017 Jun 27;8(53):90879-90896. doi: 10.18632/oncotarget.18741. eCollection 2017 Oct 31. Oncotarget. 2017. PMID: 29207610 Free PMC article.
-
Reference gene considerations for toxicological assessment of the flame retardant triphenyl phosphate in an in vitro fish embryonic model.J Appl Toxicol. 2025 Feb;45(2):288-297. doi: 10.1002/jat.4698. Epub 2024 Sep 18. J Appl Toxicol. 2025. PMID: 39295171 Free PMC article.
-
The association of organophosphate flame retardants (OPFRs) exposure on omega-3 fatty acids metabolism: evidence derived from the United States general population.Toxicol Res (Camb). 2025 Aug 17;14(4):tfaf119. doi: 10.1093/toxres/tfaf119. eCollection 2025 Aug. Toxicol Res (Camb). 2025. PMID: 40827232 Free PMC article.
-
Assessing an Overall Toxicological Implication of Nail Care Product for Occupational and Consumer Health Improvement.Iran J Public Health. 2022 May;51(5):1185-1187. doi: 10.18502/ijph.v51i5.9436. Iran J Public Health. 2022. PMID: 36407730 Free PMC article. No abstract available.
References
-
- Greaves A. K. & Letcher R. J. Comparative Body Compartment Composition and In Ovo Transfer of Organophosphate Flame Retardants in North American Great Lakes Herring Gulls. Environ. Sci. Technol. 48, 7942–7950 (2014). - PubMed
-
- Mohamed A. A. & Adrian C. Organophosphate Flame Retardants in Indoor Dust from Egypt: Implications for Human Exposure. Environ. Sci. Technol. 48, 4782–4789 (2014). - PubMed
-
- Möller A. et al. Organophosphorus flame retardants and plasticizers in airborne particles over the Northern Pacific and Indian Ocean toward the polar regions: Evidence for global occurrence. Environ. Sci. Technol. 46, 3127–3134 (2012). - PubMed
-
- Regnery J. & Puettmann W. Occurrence and fate of organophosphorus flame retardants and plasticizers in urban and remote surface waters in Germany. Water Res. 44, 4097–4104 (2010). - PubMed
-
- Regnery J., Puettmann W., Merz C. & Berthold G. Occurrence and distribution of organophosphorus flame retardants and plasticizers in anthropogenically affected groundwater. J. Environ. Monit. 13, 347–354 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

