Allergic lung inflammation promotes atherosclerosis in apolipoprotein E-deficient mice
- PMID: 26898714
- PMCID: PMC4833597
- DOI: 10.1016/j.trsl.2016.01.008
Allergic lung inflammation promotes atherosclerosis in apolipoprotein E-deficient mice
Abstract
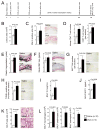
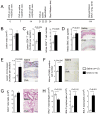
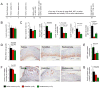
Inflammation drives asthma and atherosclerosis. Clinical studies suggest that asthmatic patients have a high risk of atherosclerosis. Yet this hypothesis remains uncertain, given that Th2 imbalance causes asthma whereas Th1 immunity promotes atherosclerosis. In this study, chronic allergic lung inflammation (ALI) was induced in mice by ovalbumin sensitization and challenge. Acute ALI was induced in mice by ovalbumin and aluminum sensitization and ovalbumin challenge. Atherosclerosis was produced in apolipoprotein E-deficient (Apoe(-/-)) mice with a Western diet. When chronic ALI and atherosclerosis were produced simultaneously, ALI increased atherosclerotic lesion size, lesion inflammatory cell content, elastin fragmentation, smooth muscle cell (SMC) loss, lesion cell proliferation, and apoptosis. Production of acute ALI before atherogenesis did not affect lesion size, but increased atherosclerotic lesion CD4(+) T cells, lesion SMC loss, angiogenesis, and apoptosis. Production of acute ALI after atherogenesis also did not change atherosclerotic lesion area, but increased lesion elastin fragmentation, cell proliferation, and apoptosis. In mice with chronic ALI and diet-induced atherosclerosis, daily inhalation of a mast cell inhibitor or corticosteroid significantly reduced atherosclerotic lesion T-cell and mast cell contents, SMC loss, angiogenesis, and cell proliferation and apoptosis, although these drugs did not affect lesion area, compared with those that received vehicle treatment. In conclusion, both chronic and acute ALI promote atherogenesis or aortic lesion pathology, regardless whether ALI occurred before, after, or at the same time as atherogenesis. Antiasthmatic medication can efficiently mitigate atherosclerotic lesion pathology.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Allergic Lung Inflammation Aggravates Angiotensin II-Induced Abdominal Aortic Aneurysms in Mice.Arterioscler Thromb Vasc Biol. 2016 Jan;36(1):69-77. doi: 10.1161/ATVBAHA.115.305911. Epub 2015 Nov 5. Arterioscler Thromb Vasc Biol. 2016. PMID: 26543094 Free PMC article.
-
Allergic asthma accelerates atherosclerosis dependent on Th2 and Th17 in apolipoprotein E deficient mice.J Mol Cell Cardiol. 2014 Jul;72:20-7. doi: 10.1016/j.yjmcc.2014.02.005. Epub 2014 Feb 14. J Mol Cell Cardiol. 2014. PMID: 24530901
-
Toll-like receptor 7 deficiency protects apolipoprotein E-deficient mice from diet-induced atherosclerosis.Sci Rep. 2017 Apr 12;7(1):847. doi: 10.1038/s41598-017-00977-0. Sci Rep. 2017. PMID: 28405010 Free PMC article.
-
Interaction between allergic asthma and atherosclerosis.Transl Res. 2016 Aug;174:5-22. doi: 10.1016/j.trsl.2015.09.009. Epub 2015 Oct 9. Transl Res. 2016. PMID: 26608212 Free PMC article. Review.
-
Crossroads between peripheral atherosclerosis, western-type diet and skeletal muscle pathophysiology: emphasis on apolipoprotein E deficiency and peripheral arterial disease.J Biomed Sci. 2017 Jul 8;24(1):42. doi: 10.1186/s12929-017-0346-8. J Biomed Sci. 2017. PMID: 28688452 Free PMC article. Review.
Cited by
-
Identification of key pathways and genes in carotid atherosclerosis through bioinformatics analysis of RNA-seq data.Aging (Albany NY). 2021 May 11;13(9):12733-12747. doi: 10.18632/aging.202943. Epub 2021 May 11. Aging (Albany NY). 2021. PMID: 33973530 Free PMC article.
-
Cellular and Molecular Mechanisms of Mast Cells in Atherosclerotic Plaque Progression and Destabilization.Clin Rev Allergy Immunol. 2024 Feb;66(1):30-49. doi: 10.1007/s12016-024-08981-9. Epub 2024 Jan 30. Clin Rev Allergy Immunol. 2024. PMID: 38289515 Free PMC article. Review.
-
Differential roles of eosinophils in cardiovascular disease.Nat Rev Cardiol. 2025 Mar;22(3):165-182. doi: 10.1038/s41569-024-01071-5. Epub 2024 Sep 16. Nat Rev Cardiol. 2025. PMID: 39285242 Review.
-
Allergic asthma is a risk factor for human cardiovascular diseases.Nat Cardiovasc Res. 2022 May;1(5):417-430. doi: 10.1038/s44161-022-00067-z. Epub 2022 May 16. Nat Cardiovasc Res. 2022. PMID: 39195946 Review.
-
Cytokines at the Interplay Between Asthma and Atherosclerosis?Front Pharmacol. 2020 Mar 4;11:166. doi: 10.3389/fphar.2020.00166. eCollection 2020. Front Pharmacol. 2020. PMID: 32194407 Free PMC article. Review.
References
-
- McFadden ER, Jr, Gilbert IA. Asthma. N Engl J Med. 1992;327:1928–37. - PubMed
-
- Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–62. - PubMed
-
- Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. - PubMed
-
- Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15. - PubMed
-
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

