A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program
- PMID: 26898779
- PMCID: PMC4790095
- DOI: 10.1016/j.neuron.2016.01.034
A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program
Abstract
The regenerative capacity of the injured CNS in adult mammals is severely limited, yet axons in the peripheral nervous system (PNS) regrow, albeit to a limited extent, after injury. We reasoned that coordinate regulation of gene expression in injured neurons involving multiple pathways was central to PNS regenerative capacity. To provide a framework for revealing pathways involved in PNS axon regrowth after injury, we applied a comprehensive systems biology approach, starting with gene expression profiling of dorsal root ganglia (DRGs) combined with multi-level bioinformatic analyses and experimental validation of network predictions. We used this rubric to identify a drug that accelerates DRG neurite outgrowth in vitro and optic nerve outgrowth in vivo by inducing elements of the identified network. The work provides a functional genomics foundation for understanding neural repair and proof of the power of such approaches in tackling complex problems in nervous system biology.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

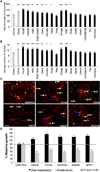

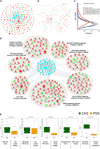
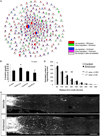
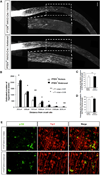
Comment in
-
Inside Out: Core Network of Transcription Factors Drives Axon Regeneration.Neuron. 2016 Mar 2;89(5):881-4. doi: 10.1016/j.neuron.2016.02.022. Neuron. 2016. PMID: 26938435
-
Neural repair: Networking for regeneration.Nat Rev Neurosci. 2016 Apr;17(4):197. doi: 10.1038/nrn.2016.29. Epub 2016 Mar 10. Nat Rev Neurosci. 2016. PMID: 26961162 No abstract available.
References
-
- Afshari FT, Kappagantula S, Fawcett JW. Extrinsic and intrinsic factors controlling axonal regeneration after spinal cord injury. Expert Rev. Mol. Med. 2009;11:e37. - PubMed
-
- Beggs S, Salter MW. Neuropathic pain: symptoms, models, and mechanisms. Drug Dev. Res. 2006;67:289–301.
-
- Bomze HM, Bulsara KR, Iskandar BJ, Caroni P, Skene JH. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat. Neurosci. 2001;4:38–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

