Phosphorylation of the amyloid β-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity
- PMID: 26898910
- PMCID: PMC4789232
- DOI: 10.1007/s00401-016-1546-0
Phosphorylation of the amyloid β-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity
Abstract
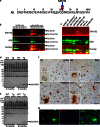
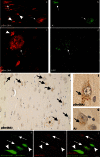
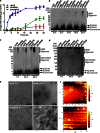
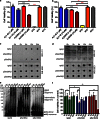
Aggregation and toxicity of the amyloid β-peptide (Aβ) are considered as critical events in the initiation and progression of Alzheimer's disease (AD). Recent evidence indicated that soluble oligomeric Aβ assemblies exert pronounced toxicity, rather than larger fibrillar aggregates that deposit in the forms of extracellular plaques. While some rare mutations in the Aβ sequence that cause early-onset AD promote the oligomerization, molecular mechanisms that induce the formation or stabilization of oligomers of the wild-type Aβ remain unclear. Here, we identified an Aβ variant phosphorylated at Ser26 residue (pSer26Aβ) in transgenic mouse models of AD and in human brain that shows contrasting spatio-temporal distribution as compared to non-phosphorylated Aβ (npAβ) or other modified Aβ species. pSer26Aβ is particularly abundant in intraneuronal deposits at very early stages of AD, but much less in extracellular plaques. pSer26Aβ assembles into a specific oligomeric form that does not proceed further into larger fibrillar aggregates, and accumulates in characteristic intracellular compartments of granulovacuolar degeneration together with TDP-43 and phosphorylated tau. Importantly, pSer26Aβ oligomers exert increased toxicity in human neurons as compared to other known Aβ species. Thus, pSer26Aβ could represent a critical species in the neurodegeneration during AD pathogenesis.
Keywords: Alzheimer’s disease; Amyloid oligomer; Granulovacuolar degeneration; Intraneuronal Abeta; Phosphorylation; Protein aggregation.
Figures
Similar articles
-
Early intraneuronal accumulation and increased aggregation of phosphorylated Abeta in a mouse model of Alzheimer's disease.Acta Neuropathol. 2013 May;125(5):699-709. doi: 10.1007/s00401-013-1107-8. Epub 2013 Mar 24. Acta Neuropathol. 2013. PMID: 23525537
-
Novel Phosphorylation-State Specific Antibodies Reveal Differential Deposition of Ser26 Phosphorylated Aβ Species in a Mouse Model of Alzheimer's Disease.Front Mol Neurosci. 2021 Jan 15;13:619639. doi: 10.3389/fnmol.2020.619639. eCollection 2020. Front Mol Neurosci. 2021. PMID: 33519377 Free PMC article.
-
Soluble Conformers of Aβ and Tau Alter Selective Proteins Governing Axonal Transport.J Neurosci. 2016 Sep 14;36(37):9647-58. doi: 10.1523/JNEUROSCI.1899-16.2016. J Neurosci. 2016. PMID: 27629715 Free PMC article.
-
Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer's disease.Acta Neuropathol. 2015 Feb;129(2):167-82. doi: 10.1007/s00401-014-1375-y. Epub 2014 Dec 23. Acta Neuropathol. 2015. PMID: 25534025 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Modified amyloid variants in pathological subgroups of β-amyloidosis.Ann Clin Transl Neurol. 2018 Jun 6;5(7):815-831. doi: 10.1002/acn3.577. eCollection 2018 Jul. Ann Clin Transl Neurol. 2018. PMID: 30009199 Free PMC article.
-
Amyloid β Proteoforms Elucidated by Quantitative LC/MS in the 5xFAD Mouse Model of Alzheimer's Disease.J Proteome Res. 2023 Nov 3;22(11):3475-3488. doi: 10.1021/acs.jproteome.3c00353. Epub 2023 Oct 17. J Proteome Res. 2023. PMID: 37847596 Free PMC article.
-
N-Terminally Truncated and Pyroglutamate-Modified Aβ Forms Are Measurable in Human Cerebrospinal Fluid and Are Potential Markers of Disease Progression in Alzheimer's Disease.Front Neurosci. 2021 Jul 29;15:708119. doi: 10.3389/fnins.2021.708119. eCollection 2021. Front Neurosci. 2021. PMID: 34393717 Free PMC article.
-
Insights Into the Mechanism of Tyrosine Nitration in Preventing β-Amyloid Aggregation in Alzheimer's Disease.Front Mol Neurosci. 2021 Feb 15;14:619836. doi: 10.3389/fnmol.2021.619836. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33658911 Free PMC article.
-
PI3K activation prevents Aβ42-induced synapse loss and favors insoluble amyloid deposit formation.Mol Biol Cell. 2020 Feb 15;31(4):244-260. doi: 10.1091/mbc.E19-05-0303. Epub 2019 Dec 26. Mol Biol Cell. 2020. PMID: 31877058 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

