Targeting Hsp70: A possible therapy for cancer
- PMID: 26898980
- PMCID: PMC5553548
- DOI: 10.1016/j.canlet.2016.01.056
Targeting Hsp70: A possible therapy for cancer
Abstract
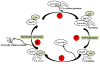
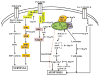
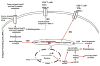
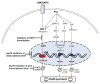
In all organisms, heat-shock proteins (HSPs) provide an ancient defense system. These proteins act as molecular chaperones by assisting proper folding and refolding of misfolded proteins and aid in the elimination of old and damaged cells. HSPs include Hsp100, Hsp90, Hsp70, Hsp40, and small HSPs. Through its substrate-binding domains, Hsp70 interacts with wide spectrum of molecules, ranging from unfolded to natively folded and aggregated proteins, and provides cytoprotective role against various cellular stresses. Under pathophysiological conditions, the high expression of Hsp70 allows cells to survive with lethal injuries. Increased Hsp70, by interacting at several points on apoptotic signaling pathways, leads to inhibition of apoptosis. Elevated expression of Hsp70 in cancer cells may be responsible for tumorigenesis and for tumor progression by providing resistance to chemotherapy. In contrast, inhibition or knockdown of Hsp70 reduces the size of tumors and can cause their complete regression. Moreover, extracellular Hsp70 acts as an immunogen that participates in cross presentation of MHC-I molecules. The goals of this review are to examine the roles of Hsp70 in cancer and to present strategies targeting Hsp70 in the development of cancer therapeutics.
Keywords: Apoptosis; Cancer therapeutics; Hsp70; Immunogenicity.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
There is no conflict of interest among the authors. The authors alone are responsible for the content and writing of this review.
Figures

References
-
- Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. - PubMed
-
- Kroeger H. The induction of new puffing patterns by transplantation of salivary gland nuclei into egg cytoplasma of Drosophila. Chromosoma. 1960;11:129–145. - PubMed
-
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. - PubMed
-
- Yahara I. Stress-inducible cellular responses. Introduction. EXS. 1996;77:XI–XII. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

