PD-1/PD-L1 blockade in cancer treatment: perspectives and issues
- PMID: 26899259
- PMCID: PMC4901122
- DOI: 10.1007/s10147-016-0959-z
PD-1/PD-L1 blockade in cancer treatment: perspectives and issues
Abstract
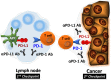
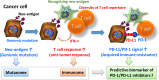
Recent studies showed that tumor cells 'edit' host immunity in several ways to evade immune defenses in the tumor microenvironment. This phenomenon is called "cancer immune escape." One of the most important components in this system is an immunosuppressive co-signal (immune checkpoint) mediated by the PD-1 receptor and its ligand, PD-L1. PD-1 is mainly expressed on activated T cells, whereas PD-L1 is expressed on several types of tumor cells. Preclinical studies have shown that inhibition of the interaction between PD-1 and PD-L1 enhances the T-cell response and mediates antitumor activity. Several clinical trials of PD-1/PD-L1 signal-blockade agents have exhibited dramatic antitumor efficacy in patients with certain types of solid or hematological malignancies. In this review, we highlight recent clinical trials using anti-PD-1 or anti-PD-L1 antibodies against several types of malignancies, including a trial conducted in our department, and describe the clinical perspectives and issues regarding the PD-1/PD-L1 blockade in cancer treatment.
Keywords: Biomarker; Immunotherapy; Nivolumab; PD-1; PD-L1; Value.
Figures
References
-
- Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

