Very early discharge versus early discharge versus non-early discharge in children with cancer and febrile neutropenia
- PMID: 26899263
- PMCID: PMC10372717
- DOI: 10.1002/14651858.CD008382.pub2
Very early discharge versus early discharge versus non-early discharge in children with cancer and febrile neutropenia
Abstract
Background: Chemotherapy-induced neutropenia is a common adverse effect in children with cancer. Due to the high relative risk of infections and infectious complications, standard care for children with cancer and febrile neutropenia consists of routine hospitalization and parenteral administration of broad-spectrum antibiotics. However, there are less serious causes of febrile neutropenia; in a subgroup of these children, lengthy in-hospital treatment might be unnecessary. Various research groups have studied the adjustment of standard care to shorten in-hospital treatment for children with cancer and febrile neutropenia at low risk for bacterial infections. However, most of these studies were not done in a randomized matter.
Objectives: To evaluate whether early discharge (mean/median of less than five days) from in-hospital treatment was not inferior to non-early discharge (mean/median of five days or more) and whether very early discharge (mean/median of less than 24 hours) was not inferior to early discharge, non-early discharge, or a combination of these, in children with cancer and febrile neutropenia.
Search methods: We searched the Cochrane Central Register of Controlled Trials (2015, issue 11), MEDLINE/PubMed (from 1945 to December 2015), EMBASE/Ovid (from 1980 to December 2015), the reference lists of relevant articles and review articles, and various conference proceedings (dependent on availability from 2005 to 2010 to 2013 to 2015). We scanned the International Standard Randomised Controlled Trials Number (ISRCTN) Register, the National Institute of Health Register for ongoing trials, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on 9 January 2016.
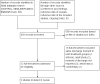
Selection criteria: We included all randomized controlled trials and controlled clinical trials in which children with cancer and febrile neutropenia were divided in groups with different times of discharge.
Data collection and analysis: We used standard methods of Cochrane and its Childhood Cancer Group. Two independent review authors performed study selection, data extraction, and risk of bias assessment. We entered data extracted from the included studies into Review Manager 5 and undertook analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results: We included two randomized controlled trials assessing very early, early, non-early (or a combination of these) discharge in children with cancer and febrile neutropenia. We graded the evidence as low quality; we downgraded for risk of bias and imprecision. One study, Santolaya 2004, consisted of 149 randomized low-risk episodes and compared early discharge (mean/median of less than five days) to non-early discharge (mean/median of five days or more). This study found no clear evidence of difference in treatment failure (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.24 to 3.50, P value = 0.89 for rehospitalization or adjustment of antimicrobial treatment, or both; Fischer's exact P value = 0.477 for death) or duration of treatment (mean difference -0.3 days, 95% CI -1.22 to 0.62, P value = 0.52 for any antimicrobial treatment; mean difference -0.5 days, 95% CI -1.36 to 0.36, P value = 0.25 for intravenous antimicrobial treatment; mean difference 0.2 days, 95% CI -0.51 to 0.91, P value = 0.58 for oral antimicrobial treatment). Costs were lower in the early discharge group (mean difference USD -265, 95% CI USD -403.14 to USD -126.86, P value = 0.0002). The second included study, Brack 2012, consisted of 62 randomized low-risk episodes and compared very early discharge (mean/median of less than 24 hours) to early discharge (mean/median of less than five days). This study also found no clear evidence of difference in treatment failure (RR 0.54, 95% CI 0.15 to 1.89, P value = 0.34 for rehospitalization or adjustment of antimicrobial treatment (or both); Fischer's exact P value = 0.557 for death). Regarding duration of treatment, median duration of intravenous antimicrobial treatment was shorter in the very early discharge group (Wilcoxon's P value ≤ 0.001, stated in the study) and median duration of oral antimicrobial treatment was shorter in the early discharge group (Wilcoxon's P ≤ 0.001, stated in the study) as compared to one another. However, there was no clear evidence of difference in median duration of any antimicrobial treatment (Wilcoxon's P value = 0.34, stated in the study). Costs were not assessed in this study. Neither of the included studies assessed quality of life. Meta-analysis was not possible as the included studies assessed different discharge moments and used different risk stratification models.
Authors' conclusions: Very limited data were available regarding the safety of early discharge compared to non-early discharge from in-hospital treatment in children with cancer and febrile neutropenia and a low risk for invasive infection. The absence of clear evidence of differences in both studies could be due to lack of power.Evidently, there are still profound gaps regarding very early and early discharge in children with cancer and febrile neutropenia. Future studies that assess this subject should have a large sample size and aim to establish uniform and objective criteria regarding the identification of a low-risk febrile neutropenic episode.
Conflict of interest statement
None known.
Figures

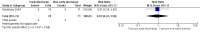

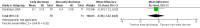
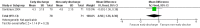
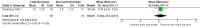
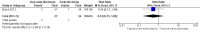
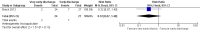







Update of
References
References to studies included in this review
Brack 2012 {published data only}
-
- Brack E, Bodmer N, Simon A, Leibundgut K, Kühne T, Niggli FK, et al. First‐day step‐down to oral outpatient treatment versus continued standard treatment in children with cancer and low‐risk fever in neutropenia. A randomized controlled trial within the multicenter SPOG 2003 FN study. Pediatric Blood & Cancer 2012;59:423‐30. - PubMed
Santolaya 2004 {published data only}
-
- Santolaya ME, Alvarez AM, Avilés CL, Becker A, Cofré J, Cumsille MA, et al. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. Journal of Clinical Oncology 2004;22(18):3784‐9. - PubMed
References to studies excluded from this review
Ahmed 2007 {published data only}
-
- Ahmed N, El‐Mahallawy HA, Ahmed IA, Nassif S, El‐Beshlawy A, El‐Haddad A. Early hospital discharge versus continued hospitalization in febrile pediatric cancer patients with prolonged neutropenia: a randomized, prospective study. Pediatric Blood & Cancer 2007;49:786‐92. - PubMed
Arora 2014 {published data only}
-
- Arora B, Banavali S, Vora T, Chinnaswamy G, Prasad M, Paradkar A, et al. A randomized open labeled parallel group phase III study of antibiotics plus G‐CSF in pediatric cancer patients with febrile neutropenia in a low‐income setting. Pediatric Blood & Cancer 2014;61 Suppl 2:S105‐433.
Feng 2014 {published data only}
-
- Feng X, Ruan Y, He Y, Zhang Y, Wu X, Liu H, et al. Prophylactic first‐line antibiotics reduce infectious fever and shorten hospital stay during chemotherapy‐induced agranulocytosis in childhood acute myeloid leukemia. Acta Haematologica 2014;132(1):112‐7. - PubMed
Georgala 2012 {published data only}
-
- Georgala A, Loizidou A, Berghmans T, Aoun M, Laethem V, Dubreucq L, et al. Feasibility of very early discharge of febrile neutropenia (FN) patients (pts) selected using the Mascc score: a Belgian study. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. 2012; Vol. T1028:110.
Gupta 2009 {published data only}
-
- Gupta A, Swaroop C, Agarwala S, Pandey RM, Bakhshi S. Randomized control trial comparing oral amoxicillin‐clavulanate and ofloxacin with intravenous ceftriaxone and amikacin as outpatient therapy in pediatric low‐risk febrile neutropenia. Journal of Pediatric Hematology‐Oncology 2009;31:635‐41. - PubMed
Kern 2013 {published data only}
-
- Kern WV, Marchetti O, Drgona L, Akan H, Aoun M, Akova M, et al. Oral antibiotics for fever in low‐risk neutropenic patients with cancer: a double‐blind, randomized, multicenter trial comparing single daily moxifloxacin with twice daily ciprofloxacin plus amoxicillin/clavulanic acid combination therapy ‐ EORTC infectious diseases group trial XV. Journal of Clinical Oncology 2013;31(9):1149‐56. - PubMed
Mathew 2014 {published data only}
-
- Mathew JL, Arora RS, Sankar J. Outpatient versus inpatient IV antibiotic management for pediatric oncology patients with low risk febrile neutropenia: a randomised trial. Indian Pediatrics 2014;51:659‐61. - PubMed
Orme 2014 {published data only}
-
- Orme LM, Babl FE, Barnes C, Barnett P, Donath S, Ashley DM. Outpatient versus inpatient IV antibiotic management for pediatric oncology patients with low risk febrile neutropenia: a randomised trial. Pediatric Blood & Cancer 2014;61(8):1427‐33. - PubMed
Paganini 2000 {published data only}
-
- Paganini HR, Sarkis CM, Martino MG, Zubizarreta PA, Casimir L, Fernandez C, et al. Oral administration of cefixime to lower risk febrile neutropenic children with cancer. Cancer 2000;88(12):2848‐52. - PubMed
Shenep 2001 {published data only}
-
- Shenep JL, Flynn PM, Baker DK, Hetherington SV, Hudson MM, Hughes WT, et al. Oral cefixime is similar to continued intravenous antibiotics in the empirical treatment of febrile neutropenic children with cancer. Clinical Infectious Diseases 2001;32(1):36‐43. - PubMed
Additional references
Ammann 2010
-
- Ammann RA, Bodmer N, Hirt A, Niggli FK, Nadal D, Simon A, et al. Predicting adverse events in children with fever and chemotherapy‐induced neutropenia: the prospective multicenter SPOG 2003 FN study. Journal of Clinical Oncology 2010;28(12):2008‐14. - PubMed
Ammann 2011
-
- Ammann RA, Hirt A, Nadal D, Simon A, Kühne T, Aebi C. Reply to K.G.E. Miedema et al. Journal of Clinical Oncology 2011;29(7):e185.
Ariffin 2006
-
- Ariffin H, Ai CL, Lee CL, Abdullah WA. Cefepime monotherapy for treatment of febrile neutropenia in children. Journal of Paediatrics and Child Health 2006;42(12):781‐4. - PubMed
Bodey 1966
-
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine 1966;64(2):328‐40. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodgson‐Viden 2005
Hughes 2002
-
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clinical Infectious Diseases 2002;34(6):730‐51. - PubMed
Klastersky 2013
-
- Klastersky J, Paesmans M. The Multinational Association for Supportive Care in Cancer (MASCC) risk index score: 10 years of use for identifying low‐risk febrile neutropenic cancer patients. Supportive Care in Cancer 2013;21:1487‐95. - PubMed
Miedema 2011
-
- Miedema KG, Bont ES, Oude Nijhuis CS, Vliet D, Kamps WA, Tissing WJ. Validation of a new risk assessment model for predicting adverse events in children with fever and chemotherapy‐induced neutropenia. Journal of Clinical Oncology 2011;29(7):e182‐4. - PubMed
Module CCG
-
- Kremer LCM, Dalen EC, Moher D, Caron HN. Childhood Cancer Group. In: The Cochrane Library, 2010, Issue 2 . Chichester: Wiley‐Blackwell. Updated quarterly.
O'Leary 2008
Oude Nijhuis 2005
-
- Oude Nijhuis C, Kamps WA, Daenen SM, Gietema JA, Graaf WT, Groen HJ, Vellenga E, Vergert EM, Vermeulen KM, Vries‐Hospers HG, Bont ES. Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients.. Journal of Clinical Oncology 2005 Oct 20;23(30):7437‐44. - PubMed
Petrilli 1993
-
- Petrilli AS, Melaragno R, Barros KV, Silva AA, Kusano E, Ribeiro RC, et al. Fever and neutropenia in children with cancer: a therapeutic approach related to the underlying disease. Pediatric Infectious Disease Journal 1993;12(11):916‐21. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2012.
Santolaya 1997
-
- Santolaya ME, Villarroel M, Avendano LF, Cofré J. Discontinuation of antimicrobial therapy for febrile, neutropenic children with cancer: a prospective study. Clinical Infectious Diseases 1997;25(1):92‐7. - PubMed
Santolaya 2002
-
- Santolaya ME, Alvarez AM, Avilés CL, Becker A, Cofré J, Enríquez N, et al. Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clinical Infectious Diseases 2002;35(6):678‐83. - PubMed
te Poele 2009
-
- Poele EM, Tissing WJ, Kamps WA, Bont ES. Risk assessment in fever and neutropenia in children with cancer: what did we learn?. Critical Reviews in Oncology/Hematology 2009;72(1):45‐55. - PubMed
Wacker 1997
-
- Wacker P, Halperin DS, Wyss M, Humbert J. Early hospital discharge of children with fever and neutropenia: a prospective study . Journal of Pediatric Hematology/Oncology 1997;19(3):208‐11. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

