High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation after spinal cord injury
- PMID: 26899371
- PMCID: PMC4761922
- DOI: 10.1038/srep21607
High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation after spinal cord injury
Abstract
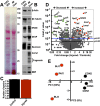
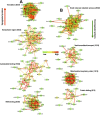
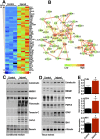
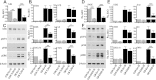
Spinal cord injury is characterized by acute cellular and axonal damage followed by aggressive inflammation and pathological tissue remodelling. The biological mediators underlying these processes are still largely unknown. Here we apply an innovative proteomics approach targeting the enriched extracellular proteome after spinal cord injury for the first time. Proteomics revealed multiple matrix proteins not previously associated with injured spinal tissue, including small proteoglycans involved in cell-matrix adhesion and collagen fibrillogenesis. Network analysis of transcriptomics and proteomics datasets uncovered persistent overexpression of extracellular alarmins that can trigger inflammation via pattern recognition receptors. In mechanistic experiments, inhibition of toll-like receptor-4 (TLR4) and the receptor for advanced glycation end-products (RAGE) revealed the involvement of alarmins in inflammatory gene expression, which was found to be dominated by IL1 and NFκΒ signalling. Extracellular high-mobility group box-1 (HMGB1) was identified as the likely endogenous regulator of IL1 expression after injury. These data reveal a novel tissue remodelling signature and identify endogenous alarmins as amplifiers of the inflammatory response that promotes tissue pathology and impedes neuronal repair after spinal cord injury.
Figures



References
-
- Benowitz L. I. & Popovich P. G. Inflammation and axon regeneration. Curr Opin Neurol 24(6), 577–583 (2011). - PubMed
-
- Silver J. & Miller J. H. Regeneration beyond the glial scar. Nat Rev Neurosci 5(2), 146–156 (2004). - PubMed
-
- Fawcett J. W. & Asher R. A. The glial scar and central nervous system repair. Brain Res Bull 49(6), 377–391 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

