High flow nasal cannula for respiratory support in preterm infants
- PMID: 26899543
- PMCID: PMC9371597
- DOI: 10.1002/14651858.CD006405.pub3
High flow nasal cannula for respiratory support in preterm infants
Update in
-
Nasal high flow therapy for primary respiratory support in preterm infants.Cochrane Database Syst Rev. 2023 May 5;5(5):CD006405. doi: 10.1002/14651858.CD006405.pub4. Cochrane Database Syst Rev. 2023. PMID: 37144837 Free PMC article. Review.
Abstract
Background: High flow nasal cannulae (HFNC) are small, thin, tapered binasal tubes that deliver oxygen or blended oxygen/air at gas flows of more than 1 L/min. HFNC are increasingly being used as a form of non-invasive respiratory support for preterm infants.
Objectives: To compare the safety and efficacy of HFNC with other forms of non-invasive respiratory support in preterm infants.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1), MEDLINE via PubMed (1966 to 1 January 2016), EMBASE (1980 to 1 January 2016), and CINAHL (1982 to 1 January 2016). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: Randomised or quasi-randomised trials comparing HFNC with other non-invasive forms of respiratory support in preterm infants immediately after birth or following extubation.
Data collection and analysis: The authors extracted and analysed data, and calculated risk ratio, risk difference and number needed to treat for an additional beneficial outcome.
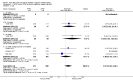
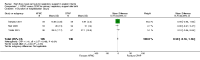
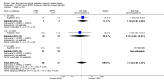
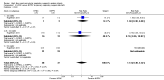
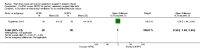
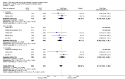
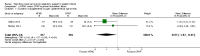
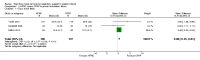
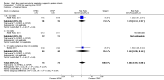
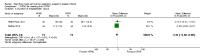
Main results: We identified 15 studies for inclusion in the review. The studies differed in the interventions compared (nasal continuous positive airway pressure (CPAP), nasal intermittent positive pressure ventilation (NIPPV), non-humidified HFNC, models for delivering HFNC), the gas flows used and the indications for respiratory support (primary support from soon after birth, post-extubation support, weaning from CPAP support). When used as primary respiratory support after birth compared to CPAP (4 studies, 439 infants), there were no differences in the primary outcomes of death (typical risk ratio (RR) 0.36, 95% CI 0.01 to 8.73; 4 studies, 439 infants) or chronic lung disease (CLD) (typical RR 2.07, 95% CI 0.64 to 6.64; 4 studies, 439 infants). HFNC use resulted in longer duration of respiratory support, but there were no differences in other secondary outcomes. One study (75 infants) showed no differences between HFNC and NIPPV as primary support. Following extubation (total 6 studies, 934 infants), there were no differences between HFNC and CPAP in the primary outcomes of death (typical RR 0.77, 95% CI 0.43 to 1.36; 5 studies, 896 infants) or CLD (typical RR 0.96, 95% CI 0.78 to 1.18; 5 studies, 893 infants). There was no difference in the rate of treatment failure (typical RR 1.21, 95% CI 0.95 to 1.55; 5 studies, 786 infants) or reintubation (typical RR 0.91, 95% CI 0.68 to 1.20; 6 studies, 934 infants). Infants randomised to HFNC had reduced nasal trauma (typical RR 0.64, 95% CI 0.51 to 0.79; typical risk difference (RD) -0.14, 95% CI -0.20 to -0.08; 4 studies, 645 infants). There was a small reduction in the rate of pneumothorax (typical RR 0.35, 95% CI 0.11 to 1.06; typical RD -0.02, 95% CI -0.03 to -0.00; 5 studies 896 infants) in infants treated with HFNC. Subgroup analysis found no difference in the rate of the primary outcomes between HFNC and CPAP in preterm infants in different gestational age subgroups, though there were only small numbers of extremely preterm and late preterm infants. One trial (28 infants) found similar rates of reintubation for humidified and non-humidified HFNC, and two other trials (100 infants) found no difference between different models of equipment used to deliver humidified HFNC. For infants weaning from non-invasive respiratory support (CPAP), two studies (149 infants) found that preterm infants randomised to HFNC had a reduced duration of hospitalisation compared with infants who remained on CPAP.
Authors' conclusions: HFNC has similar rates of efficacy to other forms of non-invasive respiratory support in preterm infants for preventing treatment failure, death and CLD. Most evidence is available for the use of HFNC as post-extubation support. Following extubation, HFNC is associated with less nasal trauma, and may be associated with reduced pneumothorax compared with nasal CPAP. Further adequately powered randomised controlled trials should be undertaken in preterm infants comparing HFNC with other forms of primary non-invasive support after birth and for weaning from non-invasive support. Further evidence is also required for evaluating the safety and efficacy of HFNC in extremely preterm and mildly preterm subgroups, and for comparing different HFNC devices.
Conflict of interest statement
Brett Manley was the first author of one of the trials included in this review. Analysis of that paper was performed by other review authors.
Figures





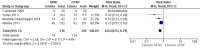

























































Update of
-
High flow nasal cannula for respiratory support in preterm infants.Cochrane Database Syst Rev. 2011 May 11;(5):CD006405. doi: 10.1002/14651858.CD006405.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2016 Feb 22;2:CD006405. doi: 10.1002/14651858.CD006405.pub3. PMID: 21563154 Updated.
References
References to studies included in this review
Abdel Hady 2011 {published and unpublished data}
-
- Abdel‐Hady H, Shouman B, Aly H. Early weaning from CPAP to high flow nasal cannula in preterm infants is associated with prolonged oxygen requirement: A randomized controlled trial. Early Human Development 2011;87(3):205‐8. [PUBMED: 21276671] - PubMed
Badiee 2015 {published data only}
Campbell 2006 {published data only}
-
- Campbell DM, Shah PS, Shah V, Kelly EN. Nasal continuous positive airway pressure from high flow cannula versus infant flow for preterm infants. Journal of Perinatology 2006;26(9):546‐9. [PUBMED: 16837929] - PubMed
Ciuffini 2014 {published data only}
-
- Ciuffini F, Pietrasanta C, Lavizzari A, Musumeci S, Gualdi C, Sortino S, et al. Comparison between two different modes of non‐invasive ventilatory support in preterm newborn infants with respiratory distress syndrome mild to moderate: preliminary data. La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics 2014;36(4):88. [PUBMED: 25573704] - PubMed
Collins 2013 {published and unpublished data}
-
- Collins CL, Barfield C, Davis PG, Horne RS. Randomized controlled trial to compare sleep and wake in preterm infants less than 32 weeks of gestation receiving two different modes of non‐invasive respiratory support. Early Human Development 2015;91(12):701‐4. [PUBMED: 26529175] - PubMed
-
- Collins CL, Barfield C, Horne RS, Davis PG. A comparison of nasal trauma in preterm infants extubated to either heated humidified high‐flow nasal cannulae or nasal continuous positive airway pressure. European Journal of Pediatrics 2014;173(2):181‐6. [PUBMED: 23955516] - PubMed
-
- Collins CL, Holberton JR, Barfield C, Davis PG. A Randomized Controlled Trial to Compare Heated Humidified High‐Flow Nasal Cannulae with Nasal Continuous Positive Airway Pressure Postextubation in Premature Infants. Journal of Pediatrics 2013;162(5):949‐54. [PUBMED: 23260098] - PubMed
Iranpour 2011 {published and unpublished data}
-
- Iranpour R, Sadeghnia A, Hesaraki M. High‐flow nasal cannula versus nasal continuous positive airway pressure in the management of respiratory distress syndrome. Journal of Isfahan Medical School 2011;29(143):1.
Kugelman 2015 {published and unpublished data}
-
- Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D. A randomized pilot study comparing heated humidified high‐flow nasal cannulae with NIPPV for RDS. Pediatric Pulmonology 2015;50(6):576‐83. [PUBMED: 24619945] - PubMed
Liu 2014 {published and unpublished data}
-
- Liu C, Collaborative Group for the Multicenter Study on Heated Humidified High‐flow Nasal Cannula Ventilation. Efficacy and safety of heated humidified high·flow nasal cannula for prevention of extubation failure in neonates [应用加温湿化高流量鼻导管通气预防 新生儿拔管失败的临床研究]. Zhonghua Er Ke Za Zhi. Chinese Journal of Pediatrics 2014;52(4):271‐6. [PUBMED: 24915914] - PubMed
Manley 2013 {published and unpublished data}
-
- Manley BJ, Owen LS, Doyle LW, Andersen CC, Cartwright DW, Pritchard MA, et al. High‐flow nasal cannulae in very preterm infants after extubation. New England Journal of Medicine 2013;369(15):1425‐33. [PUBMED: 24106935] - PubMed
Miller 2010 {published data only}
-
- Miller SM, Dowd SA. High‐flow nasal cannula and extubation success in the premature infant: a comparison of two modalities. Journal of Perinatology 2010;30(12):805‐8. [PUBMED: 20237485] - PubMed
Mostafa‐Gharehbaghi 2014 {published and unpublished data}
-
- Mostafa‐Gharehbaghi M, Mojabi H. Comparing the effectiveness of nasal continuous positive airway pressure (NCPAP) and high flow nasal cannula (HFNC) in prevention of post extubation assisted ventilation. Zahedan Journal of Research in Medical Sciences 2015;17(6):e984.
Nair 2005 {unpublished data only}
-
- Nair G, Karna P. Comparison of the effects of Vapotherm and nasal CPAP in respiratory distress. Pediatric Academic Societies Meeting; 2005 May 14‐17; Washington, DC; http://www.abstracts2view.com/pas/ (accessed May 2015):E‐PAS2005:57:2054.
Sadeghnia 2014 {published data only}
Woodhead 2006 {published data only}
-
- Woodhead DD, Lambert DK, Clark JM, Christensen RD. Comparing two methods of delivering high‐flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial. Journal of Perinatology 2006;26(8):481‐5. [PUBMED: 16724119] - PubMed
Yoder 2013 {published and unpublished data}
-
- Yoder BA, Stoddard RA, Li M, King J, Dirnberger DR, Abbasi S. Heated, humidified high‐flow nasal cannula versus nasal CPAP for respiratory support in neonates. Pediatrics 2013;131(5):e1482‐90. [PUBMED: 23610207] - PubMed
References to studies excluded from this review
Beltramo 2008 {published data only}
-
- Beltramo F, Romero R, Chandler B, Soliz A. Successful extubation in low birth weight infants: A comparison of continuous positive airway pressure (CPAP) versus Vapotherm. Pediatric Academic Society Meeting; 2008 May 3‐6; Honolulu (Hawaii); http://www.abstracts2view.com/pas/ (accessed May 2015):E‐PAS2008:63376.
Boumecid 2007 {published data only}
Capasso 2005 {published data only}
-
- Capasso L, Capasso A, Raimondi F, Vendemmia M, Araimo G, Paludetto R. A randomized trial comparing oxygen delivery on intermittent positive pressure with nasal cannulae versus facial mask in neonatal primary resuscitation. Acta Paediatrica 2005;94(2):197‐200. [PUBMED: 15981754] - PubMed
Choi 2011 {published data only}
-
- Choi BM, Lee EH, Park KH, Chung BH, Park HJ, Choi YO, et al. Comparing Usefulness of Humidified High‐Flow Nasal Cannula (HHFNC) and Nasal Continuous Positive Airway Pressure (NCPAP) for Neonatal Respiratory Diseases in Preterm Infants (Poster). Pediatric Research 2011;70:504.
Courtney 2001 {published data only}
-
- Courtney SE, Pyon KH, Saslow JG, Arnold GK, Pandit PB, Habib RH. Lung recruitment and breathing pattern during variable versus continuous flow nasal continuous positive airway pressure in premature infants: an evaluation of three devices. Pediatrics 2001;107(2):304‐8. [PUBMED: 11158463] - PubMed
de Jongh 2014 {published data only}
Fernandez‐Alvarez 2013 {published data only}
-
- Fernandez‐Alvarez JR, Gandhi RS, Amess P, Mahoney L, Watkins R, Rabe H. Heated humidified high‐flow nasal cannula versus low‐flow nasal cannula as weaning mode from nasal CPAP in infants ≤28 weeks of gestation. European Journal of Pediatrics 2014;173(1):93‐8. [PUBMED: 23942744] - PubMed
Holleman‐Duray 2007 {published data only}
-
- Holleman‐Duray D, Kaupie D, Weiss MG. Heated humidified high‐flow nasal cannula: use and a neonatal early extubation protocol. Journal of Perinatology 2007;27(12):776‐81. [PUBMED: 17855805] - PubMed
Klingenberg 2014 {published data only}
-
- Klingenberg C, Pettersen M, Hansen EA, Gustavsen LJ, Dahl IA, Leknessund A, et al. Patient comfort during treatment with heated humidified high flow nasal cannulae versus nasal continuous positive airway pressure: a randomised cross‐over trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(2):F134‐7. [PUBMED: 24225220] - PubMed
Lampland 2009 {published data only}
-
- Lampland AL, Plumm B, Meyers PA, Worwa CT, Mammel MC. Observational study of humidified high‐flow nasal cannula compared with nasal continuous positive airway pressure. The Journal of Pediatrics 2009;154(2):177‐82. [PUBMED: 18760803] - PubMed
Lavizzari 2014 {published data only}
-
- Lavizzari A, Veneroni C, Colnaghi M, Ciuffini F, Zannin E, Fumagalli M, et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(4):F315‐20. [PUBMED: 24786469] - PubMed
Mazmanyan 2013 {published data only}
-
- Mazmanyan P, Darakchyan M. Humidified high flow nasal cannula for the treatment of respiratory distress in premature newborns > 34 weeks gestation. Journal of Perinatal Medicine 2013;41.
Nasef 2015 {published data only}
-
- Nasef N, El‐Gouhary E, Schurr P, Reilly M, Beck J, Dunn M, et al. High‐flow nasal cannulae are associated with increased diaphragm activation compared with nasal continuous positive airway pressure in preterm infants. Acta Paediatrica 2015;104(8):e337‐43. [PUBMED: 25759095] - PubMed
Phadtare 2009 {published data only}
-
- Joshi R, Rajhans A, Patil S, Dominic S, Phadtare R, Devaskar U. High flow oxygen in neonatal respiratory failure: Is it better than CPAP. Pediatric Academic Society. 2008; Vol. http://www.abstracts2view.com/pas/.
-
- Phadtare R, Joshi R, Rajhans A, Patil S, Dominic S, Devaskar U. High flow nasal cannula oxygen (Vapotherm) in premature infants with respiratory distress syndrome: is it better than the conventional nasal continuous positive airways pressure (CPAP)?. Perinatology 2009;11(1):1‐8.
Pyon 2008 {published data only}
-
- Pyon KH, Aghai ZH, Nakhla TA, Stahl GE, Saslow JG. High flow nasal cannula in preterm infants: Effects of high flow rates on work of breathing. Proceedings of the Pediatric Academic Societies Annual Meeting; 2008 May 3‐6; Honolulu (HI). 2008:E‐PAS2008:633763.13.
Saslow 2006 {published data only}
-
- Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, et al. Work of breathing using high‐flow nasal cannula in preterm infants. Journal of Perinatology 2006;26(8):476‐80. [PUBMED: 16688202] - PubMed
Shoemaker 2007 {published data only}
-
- Shoemaker MT, Pierce MR, Yoder BA, DiGeronimo RJ. High flow nasal cannula versus nasal CPAP for neonatal respiratory disease: a retrospective study. Journal of Perinatology 2007;27(2):85‐91. [PUBMED: 17262040] - PubMed
Sreenan 2001 {published data only}
-
- Sreenan C, Lemke RP, Hudson‐Mason A, Osiovich H. High‐flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001;107(5):1081‐3. [PUBMED: 11331690] - PubMed
Wilson 1996 {published data only}
-
- Wilson J, Arnold C, Connor R, Cusson R. Evaluation of oxygen delivery with the use of nasopharyngeal catheters and nasal cannulas. Neonatal Network 1996;15(4):15‐22. [PUBMED: 8716524] - PubMed
References to studies awaiting assessment
Chen 2015 {published data only}
-
- Chen J, Gao WW, Xu F, Du LL, Zhang T, Ling X, Li WT. Comparison of clinical efficacy of heated humidified high flow nasal cannula versus nasal continuous positive airway pressure in treatment of respiratory distress syndrome in very low birth weight infants. Zhongguo Dang Dai Er Ke Za Zhi 2015;17(8):847‐51. [PUBMED: 26287351] - PubMed
Febre 2015 {published data only}
-
- Febre A, Merritt TA, Terry M, Tong C, Goldstein M. Adaptive Dynamic Inspiratory Nasal Apparatus: Comparison to Traditional Nasal Continuous Airway Pressure (NCPAP). Newborn and Infant Nursing Reviews 2015;15(1):17‐20.
Lawrence 2012 {published data only}
-
- Lawrence JR, Martin GC. A Pilot Study To Evaluate the Safety and Efficacy of High Flow Nasal Cannula vs. Conventional NCPAP. Pediatric Academic Society. 2012; Vol. http://www.abstracts2view.com/pas/.
Tang 2015 {published data only}
-
- Lutz TL, Tang J, Osborn DA, Malcolm GA, Reid S, Oliver S. High flow nasal cannula for weaning preterm infants from continuous positive airway pressure. Pediatric Academic Society Meeting; 2013 May 4‐7; Washington, DC; http://www.abstracts2view.com/pas/ (accessed May 2015):E‐PAS2013:3800.38.
References to ongoing studies
ACTRN12610000677000 {published data only}
-
- ACTRN12610000677000. High flow support versus continuous positive airway pressure (cpap) support in non‐acute respiratory support for preterm infants from 30 weeks corrected gestation. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12610000677000.
ACTRN12613000303741 {published data only}
-
- ACTRN12613000303741. A multi‐centre, randomised, controlled, non‐inferiority trial comparing high flow nasal cannulae to nasal continuous positive airway pressure as primary respiratory support, in preterm infants of 28 weeks' gestation and above, with early respiratory distress or apnoea, assessing assigned treatment failure within 72 hours. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613000303741.
ACTRN12615000077561 {published data only}
-
- ACTRN12615000077561. Weaning preterm infants with a gestational age (GA) of < 30 weeks from respiratory support: a comparison of duration of respiratory support with heated humidified high flow nasal cannula (HHHFNC) and continuous positive airway pressure (CPAP). http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12615000077561.
IRCT2014012716376N1 {published data only}
-
- IRCT2014012716376N1. Comparing two methods of cannula nasal with high flow and conventional FiO2 for successful weaning of preterm infants with respiratory distress from Nasal CPAP in Alzahra and Shahidbeheshti hospitals, Isfahan. http://www.irct.ir/index.php.
ISRCTN66716753 {published data only}
-
- ISRCTN66716753. High Flow Nasal Prongs (HFNP) therapy versus Nasal Continuous Positive Airway Pressure (NCPAP) in establishing full oral feeds in Very Low Birth Weight (VLBW) infants ‐ randomized controlled trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN66716753.
JPRN‐UMIN000013906 {published data only}
-
- JPRN‐UMIN000013906. A randomized controlled trial to compare high‐flow nasal cannula with nasal cpap after extubation in preterm infants. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000013906.
NCT01270581 {unpublished data only}
-
- High Flow Nasal Cannula vs Bubble Nasal CPAP for the Treatment of Transient Tachypnea of the Newborn in Infants > 35 Weeks Gestation. Ongoing study July 2010.
NCT01939067 {published data only}
-
- NCT01939067. Pulmonary mechanics in preterm infants treated with heated humidified high flow nasal cannula as compared to nasal continuous positive airway Pressure. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01939067.
NCT02055339 {published data only}
-
- NCT02055339. Comparison of nasal continuous positive airway pressure with low flow oxygen versus heated, humidified high flow nasal cannula for oral feeding of the premature infant (chomp trial): a pilot study. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02055339.
Additional references
Bell 1978
De Paoli 2003
De Paoli 2008
Dysart 2009
-
- Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respiratory Medicine 2009;103(10):1400‐5. [PUBMED: 19467849] - PubMed
Finer 2005
-
- Finer NN. Nasal cannula use in the preterm infant: oxygen or pressure?. Pediatrics 2005;116(5):1216‐7. [PUBMED: 16264009] - PubMed
Frey 2001
-
- Frey B, McQuillan PJ, Shann F, Freezer N. Nasopharyngeal oxygen therapy produces positive end‐expiratory pressure in infants. European Journal of Pediatrics 2001;160(9):556‐60. [PUBMED: 11585079] - PubMed
Frey 2003
Frizzola 2011
Garland 1985
-
- Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics 1985;76(3):406‐10. [PUBMED: 4034300] - PubMed
Hegde 2013
-
- Hegde S, Prodhan P. Serious Air Leak Syndrome Complicating High‐Flow Nasal Cannula Therapy: A Report of 3 Cases. Pediatrics 2013;131(3):e939‐44. [PUBMED: 23382446] - PubMed
Hensey 2013
-
- Hensey CC, Hayden E, O'Donnell CPF. A randomised crossover study of low‐flow air or oxygen via nasal cannulae to prevent desaturation in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(5):F388‐91. [PUBMED: 23315286] - PubMed
Hough 2012
-
- Hough JL, Shearman AD, Jardine LA, Davies MW. Humidified high flow nasal cannulae: Current practice in Australasian nurseries, a survey. Journal of Paediatrics and Child Health 2012;48(2):106‐13. [PUBMED: 21470336] - PubMed
Jasin 2008
-
- Jasin LR, Kern S, Thompson S, Walter C, Rone JM, Yohannan MD. Subcutaneous scalp emphysema, pneumo‐orbitis and pneumocephalus in a neonate on high humidity high flow nasal cannula. Journal of Perinatology 2008;28(11):779‐81. [PUBMED: 18974751] - PubMed
Jobe 2001
-
- Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [PUBMED: 11401896] - PubMed
Kopelman 2003a
-
- Kopelman AE. Airway obstruction in two extremely low birthweight infants treated with oxygen cannulas. Journal of Perinatology 2003;23(2):164‐5. [PUBMED: 12673269] - PubMed
Kopelman 2003b
-
- Kopelman AE, Holbert D. Use of oxygen cannulas in extremely low birthweight infants is associated with mucosal trauma and bleeding, and possibly with coagulase‐negative staphylococcal sepsis. Journal of Perinatology 2003;23(2):94‐7. [PUBMED: 12673256] - PubMed
Manley 2012
-
- Manley BJ, Owen L, Doyle LW, Davis PG. High‐flow nasal cannulae and nasal continuous positive airway pressure use in non‐tertiary special care nurseries in Australia and New Zealand. Journal of Paediatrics and Child Health 2012;48(1):16‐21. [PUBMED: 21988616] - PubMed
Morley 2004
-
- Morley C, Davis P. Continuous positive airway pressure: current controversies. Current Opinion in Pediatrics 2004;16(2):141‐5. [PUBMED: 15021191] - PubMed
O'Donnell 2013
-
- O'Donnell SM, Curry SJ, Buggy NA, Moynihan MM, Sebkova S, Janota J, et al. The NOFLO trial: low‐flow nasal prongs therapy in weaning nasal continuous positive airway pressure in preterm infants. Journal of Pediatrics 2013;163(1):79‐83. [PUBMED: 23312683] - PubMed
Osman 2014
-
- Osman M, Elsharkawy A, Abdel‐Hady H. Assessment of pain during application of nasal continuous positive airway pressure and heated, humidified high‐flow nasal cannulae in preterm infants. Journal of Perinatology 2014 2015;35(4):263‐7. [PUBMED: 25429383] - PubMed
Roberts 2014
-
- Roberts CT, Manley BJ, Dawson JA, Davis PG. Nursing perceptions of high‐flow nasal cannulae treatment for very preterm infants. Journal of Paediatrics and Child Health 2014;50(10):806‐10. [PUBMED: 24943729] - PubMed
Robertson 1996
Spence 2007
-
- Spence KL, Murphy D, Kilian C, McGonigle R, Kilani RA. High‐flow nasal cannula as a device to provide continuous positive airway pressure in infants. Journal of Perinatology 2007;27(12):772‐5. [PUBMED: 17762844] - PubMed
Walsh 2005
-
- Walsh M, Engle W, Laptook A, Kazzi SN, Buchter S, Rasmussen M, et al. Oxygen delivery through nasal cannulae to preterm infants: can practice be improved?. Pediatrics 2005;116(4):857‐61. [PUBMED: 16199694] - PubMed
Wilkinson 2008
-
- Wilkinson DJ, Andersen CC, Smith K, Holberton J. Pharyngeal pressure with high‐flow nasal cannulae in premature infants. Journal of Perinatology 2008;28(1):42‐7. [PUBMED: 17989697] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

