Xeno-sensing activity of the aryl hydrocarbon receptor in human pluripotent stem cell-derived hepatocyte-like cells
- PMID: 26899675
- PMCID: PMC4761945
- DOI: 10.1038/srep21684
Xeno-sensing activity of the aryl hydrocarbon receptor in human pluripotent stem cell-derived hepatocyte-like cells
Abstract
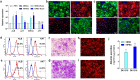
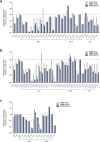
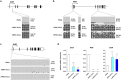
Although hepatocyte-like cells derived from human pluripotent stem cells (hPSC-HLCs) are considered a promising model for predicting hepatotoxicity, their application has been restricted because of the low activity of drug metabolizing enzymes (DMEs). Here we found that the low expression of xenobiotic receptors (constitutive androstane receptor, CAR; and pregnane X receptor, PXR) contributes to the low activity of DMEs in hPSC-HLCs. Most CAR- and PXR-regulated DMEs and transporters were transcriptionally down-regulated in hPSC-HLC. Transcriptional expression of CAR and PXR was highly repressed in hPSC-HLCs, whereas mRNA levels of aryl hydrocarbon receptor (AHR) were comparable to those of adult liver. Furthermore, ligand-induced transcriptional activation was observed only at AHR in hPSC-HLCs. Bisulfite sequencing analysis demonstrated that promoter hypermethylation of CAR and PXR was associated with diminished transcriptional activity in hPSC-HLCs. Treatment with AHR-selective ligands increased the transcription of AHR-dependent target genes by direct AHR-DNA binding at the xenobiotic response element. In addition, an antagonist of AHR significantly inhibited AHR-dependent target gene expression. Thus, AHR may function intrinsically as a xenosensor as well as a ligand-dependent transcription factor in hPSC-HLCs. Our results indicate that hPSC-HLCs can be used to screen toxic substances related to AHR signaling and to identify potential AHR-targeted therapeutics.
Figures




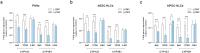
Similar articles
-
Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways.Pharm Res. 2009 Apr;26(4):872-82. doi: 10.1007/s11095-008-9788-8. Epub 2008 Nov 26. Pharm Res. 2009. PMID: 19034627 Free PMC article.
-
RNA-Seq reveals common and unique PXR- and CAR-target gene signatures in the mouse liver transcriptome.Biochim Biophys Acta. 2016 Sep;1859(9):1198-1217. doi: 10.1016/j.bbagrm.2016.04.010. Epub 2016 Apr 23. Biochim Biophys Acta. 2016. PMID: 27113289 Free PMC article.
-
Establishing Transcriptional Signatures to Differentiate PXR-, CAR-, and AhR-Mediated Regulation of Drug Metabolism and Transport Genes in Cryopreserved Human Hepatocytes.J Pharmacol Exp Ther. 2018 May;365(2):262-271. doi: 10.1124/jpet.117.247296. Epub 2018 Feb 12. J Pharmacol Exp Ther. 2018. PMID: 29440451
-
Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR.Adv Drug Deliv Rev. 2010 Oct 30;62(13):1238-49. doi: 10.1016/j.addr.2010.08.006. Epub 2010 Aug 17. Adv Drug Deliv Rev. 2010. PMID: 20727377 Free PMC article. Review.
-
Mechanisms of xenobiotic receptor activation: Direct vs. indirect.Biochim Biophys Acta. 2016 Sep;1859(9):1130-1140. doi: 10.1016/j.bbagrm.2016.02.006. Epub 2016 Feb 10. Biochim Biophys Acta. 2016. PMID: 26877237 Free PMC article. Review.
Cited by
-
Phthalazinone Pyrazole Enhances the Hepatic Functions of Human Embryonic Stem Cell-Derived Hepatocyte-Like Cells via Suppression of the Epithelial-Mesenchymal Transition.Stem Cell Rev Rep. 2018 Jun;14(3):438-450. doi: 10.1007/s12015-017-9795-4. Stem Cell Rev Rep. 2018. PMID: 29238913
-
Development of genetic quality tests for good manufacturing practice-compliant induced pluripotent stem cells and their derivatives.Sci Rep. 2020 Mar 3;10(1):3939. doi: 10.1038/s41598-020-60466-9. Sci Rep. 2020. PMID: 32127560 Free PMC article.
-
Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target.Arch Toxicol. 2017 Jul;91(7):2497-2513. doi: 10.1007/s00204-017-1981-2. Epub 2017 May 15. Arch Toxicol. 2017. PMID: 28508231 Free PMC article. Review.
-
A liver-specific gene expression panel predicts the differentiation status of in vitro hepatocyte models.Hepatology. 2017 Nov;66(5):1662-1674. doi: 10.1002/hep.29324. Epub 2017 Oct 3. Hepatology. 2017. PMID: 28640507 Free PMC article.
-
Assessing bioactivity of environmental water samples filtered using nanomembrane technology and mammalian cell lines.Eco Environ Health. 2024 May 29;3(3):347-354. doi: 10.1016/j.eehl.2024.05.004. eCollection 2024 Sep. Eco Environ Health. 2024. PMID: 39281073 Free PMC article.
References
-
- Martignoni M., Groothuis G. M. & de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2, 875–94 (2006). - PubMed
-
- Cohen A. J. Critical review of the toxicology of coumarin with special reference to interspecies differences in metabolism and hepatotoxic response and their significance to man. Food Cosmet Toxicol. 17, 277–89 (1979). - PubMed
-
- Gomez-Lechon M. J., Donato M. T., Castell J. V. & Jover R. Human hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr Drug Metab. 5, 443–62 (2004). - PubMed
-
- Hewitt N. J. et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 39, 159–234 (2007). - PubMed
-
- Guguen-Guillouzo C. & Guillouzo A. General review on in vitro hepatocyte models and their applications. Methods Mol Biol. 640, 1–40 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

