Autophagy-Modulated Human Bone Marrow-Derived Mesenchymal Stem Cells Accelerate Liver Restoration in Mouse Models of Acute Liver Failure
- PMID: 26899739
- PMCID: PMC4949977
- DOI: 10.7508/ibj.2016.03.002
Autophagy-Modulated Human Bone Marrow-Derived Mesenchymal Stem Cells Accelerate Liver Restoration in Mouse Models of Acute Liver Failure
Abstract
Background: Mesenchymal stem cells (MSCs) have been recently received increasing attention for cell-based therapy, especially in regenerative medicine. However, the low survival rate of these cells restricts their therapeutic applications. It is hypothesized that autophagy might play an important role in cellular homeostasis and survival. This study aims to investigate the regenerative potentials of autophagy-modulated MSCs for the treatment of acute liver failure (ALF) in mice.
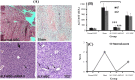
Methods: ALF was induced in mice by intraperitoneal injection of 1.5 ml/kg carbon tetrachloride. Mice were intravenously infused with MSCs, which were suppressed in their autophagy pathway. Blood and liver samples were collected at different intervals (24, 48 and 72 h) after the transplantation of MSCs. Both the liver enzymes and tissue necrosis levels were evaluated using biochemical and histopathological assessments. The survival rate of the transplanted mice was also recorded during one week.
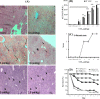
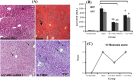
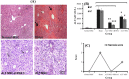
Results: Biochemical and pathological results indicated that 1.5 ml/kg carbon tetrachloride induces ALF in mice. A significant reduction of liver enzymes and necrosis score were observed in autophagy-modulated MSC-transplanted mice compared to sham (with no cell therapy) after 24 h. After 72 h, liver enzymes reached their normal levels in mice transplanted with autophagy-suppressed MSCs. Interestingly, normal histology without necrosis was also observed.
Conclusion: Autophagy suppression in MSCs ameliorates their liver regeneration potentials due to paracrine effects and might be suggested as a new strategy for the improvement of cell therapy in ALF.
Keywords: Acute liver failure; Autophagy; Mesenchymal stem cells.
Figures

Similar articles
-
Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure.J Cell Biochem. 2018 Jul;119(7):5834-5842. doi: 10.1002/jcb.26772. Epub 2018 Mar 25. J Cell Biochem. 2018. PMID: 29575235
-
Useful properties of undifferentiated mesenchymal stromal cells and adipose tissue as the source in liver-regenerative therapy studied in an animal model of severe acute fulminant hepatitis.Cytotherapy. 2015 Aug;17(8):1052-65. doi: 10.1016/j.jcyt.2015.04.010. Cytotherapy. 2015. PMID: 26139545
-
Mesenchymal stem cells increase heme oxygenase 1-activated autophagy in treatment of acute liver failure.Biochem Biophys Res Commun. 2019 Jan 15;508(3):682-689. doi: 10.1016/j.bbrc.2018.11.146. Epub 2018 Dec 5. Biochem Biophys Res Commun. 2019. PMID: 30528392
-
Progress in mesenchymal stem cell-based therapy for acute liver failure.Stem Cell Res Ther. 2018 Aug 24;9(1):227. doi: 10.1186/s13287-018-0972-4. Stem Cell Res Ther. 2018. PMID: 30143052 Free PMC article. Review.
-
[Application of mesenchymal stem cells to liver regenerative medicine].Yakugaku Zasshi. 2008 Jan;128(1):3-9. doi: 10.1248/yakushi.128.3. Yakugaku Zasshi. 2008. PMID: 18176050 Review. Japanese.
Cited by
-
A comprehensive investigation on liver regeneration: a meta-analysis and systems biology approach.Clin Exp Hepatol. 2021 Jun;7(2):183-190. doi: 10.5114/ceh.2021.107564. Epub 2021 Jun 30. Clin Exp Hepatol. 2021. PMID: 34295986 Free PMC article.
-
Human umbilical cord MSC-derived hepatocyte growth factor enhances autophagy in AOPP-treated HK-2 cells.Exp Ther Med. 2020 Sep;20(3):2765-2773. doi: 10.3892/etm.2020.8998. Epub 2020 Jul 13. Exp Ther Med. 2020. PMID: 32765771 Free PMC article.
-
Bone-Marrow-Derived Mesenchymal Stem Cells, Their Conditioned Media, and Olive Leaf Extract Protect against Cisplatin-Induced Toxicity by Alleviating Oxidative Stress, Inflammation, and Apoptosis in Rats.Toxics. 2022 Sep 6;10(9):526. doi: 10.3390/toxics10090526. Toxics. 2022. PMID: 36136492 Free PMC article.
-
Dual Preconditioning: A Novel Strategy to Withstand Mesenchymal Stem Cells against Harsh Microenvironments.Adv Pharm Bull. 2018 Aug;8(3):465-470. doi: 10.15171/apb.2018.054. Epub 2018 Aug 29. Adv Pharm Bull. 2018. PMID: 30276143 Free PMC article.
-
Transplantation of Mesenchymal Stem Cells Attenuates Acute Liver Failure in Mice via an Interleukin-4-dependent Switch to the M2 Macrophage Anti-inflammatory Phenotype.J Clin Transl Hepatol. 2022 Aug 28;10(4):669-679. doi: 10.14218/JCTH.2021.00127. Epub 2022 Jan 4. J Clin Transl Hepatol. 2022. PMID: 36062289 Free PMC article.
References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
-
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell stem cell. 2008;2(2):141–150. - PubMed
-
- Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–427. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
