Smart Cup: A Minimally-Instrumented, Smartphone-Based Point-of-Care Molecular Diagnostic Device
- PMID: 26900258
- PMCID: PMC4756427
- DOI: 10.1016/j.snb.2016.01.073
Smart Cup: A Minimally-Instrumented, Smartphone-Based Point-of-Care Molecular Diagnostic Device
Abstract
Nucleic acid amplification-based diagnostics offer rapid, sensitive, and specific means for detecting and monitoring the progression of infectious diseases. However, this method typically requires extensive sample preparation, expensive instruments, and trained personnel. All of which hinder its use in resource-limited settings, where many infectious diseases are endemic. Here, we report on a simple, inexpensive, minimally-instrumented, smart cup platform for rapid, quantitative molecular diagnostics of pathogens at the point of care. Our smart cup takes advantage of water-triggered, exothermic chemical reaction to supply heat for the nucleic acid-based, isothermal amplification. The amplification temperature is regulated with a phase-change material (PCM). The PCM maintains the amplification reactor at a constant temperature, typically, 60-65°C, when ambient temperatures range from 12 to 35°C. To eliminate the need for an optical detector and minimize cost, we use the smartphone's flashlight to excite the fluorescent dye and the phone camera to record real-time fluorescence emission during the amplification process. The smartphone can concurrently monitor multiple amplification reactors and analyze the recorded data. Our smart cup's utility was demonstrated by amplifying and quantifying herpes simplex virus type 2 (HSV-2) with LAMP assay in our custom-made microfluidic diagnostic chip. We have consistently detected as few as 100 copies of HSV-2 viral DNA per sample. Our system does not require any lab facilities and is suitable for use at home, in the field, and in the clinic, as well as in resource-poor settings, where access to sophisticated laboratories is impractical, unaffordable, or nonexistent.
Keywords: Chemical heating; HSV-2 virus detection; Loop mediated isothermal amplification; Smartphone; microfluidics.
Figures


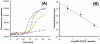
References
-
- Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–418. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
