Blocking MHC class II on human endothelium mitigates acute rejection
- PMID: 26900601
- PMCID: PMC4756651
- DOI: 10.1172/jci.insight.85293
Blocking MHC class II on human endothelium mitigates acute rejection
Abstract
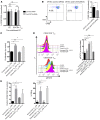
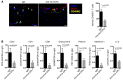
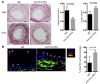
Acute allograft rejection is mediated by host CD8+ cytotoxic T lymphocytes (CTL) targeting graft class I major histocompatibility complex (MHC) molecules. In experimental rodent models, rejection requires differentiation of naive CD8+ T cells into alloreactive CTL within secondary lymphoid organs, whereas in humans, CTL may alternatively develop within the graft from circulating CD8+ effector memory T cells (TEM) that recognize class I MHC molecules on graft endothelial cells (EC). This latter pathway is poorly understood. Here, we show that host CD4+ TEM, activated by EC class II MHC molecules, provide critical help for this process. First, blocking HLA-DR on EC lining human artery grafts in immunodeficient mice reduces CD8+ CTL development within and acute rejection of the artery by adoptively transferred allogeneic human lymphocytes. Second, siRNA knockdown or CRISPR/Cas9 ablation of class II MHC molecules on EC prevents CD4+ TEM from helping CD8+ TEM to develop into CTL in vitro. Finally, implanted synthetic microvessels, formed from CRISPR/Cas9-modified EC lacking class II MHC molecules, are significantly protected from CD8+ T cell-mediated destruction in vivo. We conclude that human CD8+ TEM-mediated rejection targeting graft EC class I MHC molecules requires help from CD4+ TEM cells activated by recognition of class II MHC molecules.
Figures




References
-
- Colvin RB, et al. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J Am Soc Nephrol. 1997;8(12):1930–1941. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

