Functional Rescue of Trafficking-Impaired ABCB4 Mutants by Chemical Chaperones
- PMID: 26900700
- PMCID: PMC4764328
- DOI: 10.1371/journal.pone.0150098
Functional Rescue of Trafficking-Impaired ABCB4 Mutants by Chemical Chaperones
Abstract
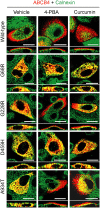
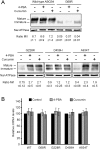
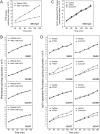
Multidrug resistance protein 3 (MDR3, ABCB4) is a hepatocellular membrane protein that mediates biliary secretion of phosphatidylcholine. Null mutations in ABCB4 gene give rise to severe early-onset cholestatic liver disease. We have previously shown that the disease-associated mutations p.G68R, p.G228R, p.D459H, and p.A934T resulted in retention of ABCB4 in the endoplasmic reticulum, thus failing to target the plasma membrane. In the present study, we tested the ability of two compounds with chaperone-like activity, 4-phenylbutyrate and curcumin, to rescue these ABCB4 mutants by assessing their effects on subcellular localization, protein maturation, and phospholipid efflux capability. Incubation of transfected cells at a reduced temperature (30°C) or exposure to pharmacological doses of either 4-PBA or curcumin restored cell surface expression of mutants G228R and A934T. The delivery of these mutants to the plasma membrane was accompanied by a switch in the ratio of mature to inmature protein forms, leading to a predominant expression of the mature protein. This effect was due to an improvement in the maturation rate and not to the stabilization of the mature forms. Both mutants were also functionally rescued, displaying bile salt-dependent phospholipid efflux activity after addition of 4-PBA or curcumin. Drug-induced rescue was mutant specific, given neither 4-PBA nor curcumin had an effect on the ABCB4 mutants G68R and A934T. Collectively, these data indicate that the functionality of selected trafficking-defective ABCB4 mutants can be recovered by chemical chaperones through restoration of membrane localization, suggesting a potential treatment for patients carrying such mutations.
Conflict of interest statement
Figures


References
-
- Ruetz S, Gros P. Phosphatidylcholine translocase: a physiological role for the mrd2 gene. Cell 1993; 77: 1071–1081. - PubMed
-
- Smith AJ, Timmermans-Hereijgers JL, Roelofsen B, Wirtz KW, van Blitterswijk WJ, Smit JJ, et al. The human MDR3 P-glycoprotein promotes translocation of phosphatidylcholine through the plasma membrane of fibroblasts from transgenic mice. FEBS Lett. 1994; 354: 263–266. - PubMed
-
- van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, et al. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P glycoprotein specifically translocates phosphatidylcholine. Cell 1996; 87: 507–517. - PubMed
-
- Oude Elferink RP, Paulusma CC. Function and pathophysiological importance of ABCB4 (MDR3 P-glycoprotein). Pflugers Arch. 2006; 453: 601–610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

