Cell-Penetrating, Guanidinium-Rich Oligophosphoesters: Effective and Versatile Molecular Transporters for Drug and Probe Delivery
- PMID: 26900771
- PMCID: PMC5351964
- DOI: 10.1021/jacs.5b13452
Cell-Penetrating, Guanidinium-Rich Oligophosphoesters: Effective and Versatile Molecular Transporters for Drug and Probe Delivery
Abstract
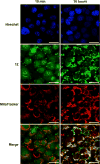
The design, synthesis, and biological evaluation of a new family of highly effective cell-penetrating molecular transporters, guanidinium-rich oligophosphoesters, are described. These unique transporters are synthesized in two steps, irrespective of oligomer length, by the organocatalytic ring-opening polymerization (OROP) of 5-membered cyclic phospholane monomers followed by oligomer deprotection. Varying the initiating alcohol results in a wide variety of cargo attachment strategies for releasable or nonreleasable transporter applications. Initiation of oligomerization with a fluorescent probe produces, upon deprotection, a transporter-probe conjugate that is shown to readily enter multiple cell lines in a dose-dependent manner. These new transporters are superior in cell uptake to previously studied guanidinium-rich oligocarbonates and oligoarginines, showing over 2-fold higher uptake than the former and 6-fold higher uptake than the latter. Initiation with a protected thiol gives, upon deprotection, thiol-terminated transporters which can be thiol-click conjugated to a variety of probes, drugs and other cargos as exemplified by the conjugation and delivery of the model probe fluorescein-maleimide and the medicinal agent paclitaxel (PTX) into cells. Of particular significance given that drug resistance is a major cause of chemotherapy failure, the PTX-transporter conjugate, designed to evade Pgp export and release free PTX after cell entry, shows efficacy against PTX-resistant ovarian cancer cells. Collectively this study introduces a new and highly effective class of guanidinium-rich cell-penetrating transporters and methodology for their single-step conjugation to drugs and probes, and demonstrates that the resulting drug/probe-conjugates readily enter cells, outperforming previously reported guanidinium-rich oligocarbonates and peptide transporters.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


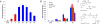

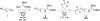
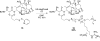
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

