Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway
- PMID: 26900866
- PMCID: PMC4798901
- DOI: 10.1038/nchembio.2036
Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway
Abstract
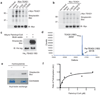
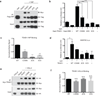
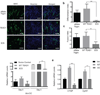
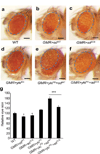
TEA domain (TEAD) transcription factors bind to the coactivators YAP and TAZ and regulate the transcriptional output of the Hippo pathway, playing critical roles in organ size control and tumorigenesis. Protein S-palmitoylation attaches a fatty acid, palmitate, to cysteine residues and regulates protein trafficking, membrane localization and signaling activities. Using activity-based chemical probes, we discovered that human TEADs possess intrinsic palmitoylating enzyme-like activities and undergo autopalmitoylation at evolutionarily conserved cysteine residues under physiological conditions. We determined the crystal structures of lipid-bound TEADs and found that the lipid chain of palmitate inserts into a conserved deep hydrophobic pocket. Strikingly, palmitoylation did not alter TEAD's localization, but it was required for TEAD's binding to YAP and TAZ and was dispensable for its binding to the Vgll4 tumor suppressor. Moreover, palmitoylation-deficient TEAD mutants impaired TAZ-mediated muscle differentiation in vitro and tissue overgrowth mediated by the Drosophila YAP homolog Yorkie in vivo. Our study directly links autopalmitoylation to the transcriptional regulation of the Hippo pathway.
Figures


References
-
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature reviews. Cancer. 2013;13:246–257. - PubMed
-
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. - PubMed
-
- Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. - PubMed
-
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

