A Novel Synthetic TLR-4 Agonist Adjuvant Increases the Protective Response to a Clinical-Stage West Nile Virus Vaccine Antigen in Multiple Formulations
- PMID: 26901122
- PMCID: PMC4762984
- DOI: 10.1371/journal.pone.0149610
A Novel Synthetic TLR-4 Agonist Adjuvant Increases the Protective Response to a Clinical-Stage West Nile Virus Vaccine Antigen in Multiple Formulations
Abstract
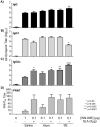
West Nile virus (WNV) is a mosquito-transmitted member of the Flaviviridae family that has emerged in recent years to become a serious public health threat. Given the sporadic nature of WNV epidemics both temporally and geographically, there is an urgent need for a vaccine that can rapidly provide effective immunity. Protection from WNV infection is correlated with antibodies to the viral envelope (E) protein, which encodes receptor binding and fusion functions. Despite many promising E-protein vaccine candidates, there are currently none licensed for use in humans. This study investigates the ability to improve the immunogenicity and protective capacity of a promising clinical-stage WNV recombinant E-protein vaccine (WN-80E) by combining it with a novel synthetic TLR-4 agonist adjuvant. Using the murine model of WNV disease, we find that inclusion of a TLR-4 agonist in either a stable oil-in-water emulsion (SE) or aluminum hydroxide (Alum) formulation provides both dose and dosage sparing functions, whereby protection can be induced after a single immunization containing only 100 ng of WN-80E. Additionally, we find that inclusion of adjuvant with a single immunization reduced viral titers in sera to levels undetectable by viral plaque assay. The enhanced protection provided by adjuvanted immunization correlated with induction of a Th1 T-cell response and the resultant shaping of the IgG response. These findings suggest that inclusion of a next generation adjuvant may greatly enhance the protective capacity of WNV recombinant subunit vaccines, and establish a baseline for future development.
Conflict of interest statement
Figures





Similar articles
-
Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection.J Immunol. 2007 Mar 1;178(5):2699-705. doi: 10.4049/jimmunol.178.5.2699. J Immunol. 2007. PMID: 17312111
-
Matrix-M™ adjuvanted envelope protein vaccine protects against lethal lineage 1 and 2 West Nile virus infection in mice.Vaccine. 2014 Feb 7;32(7):800-8. doi: 10.1016/j.vaccine.2013.12.030. Epub 2013 Dec 28. Vaccine. 2014. PMID: 24380682
-
A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity.J Infect Dis. 2007 Jun 1;195(11):1607-17. doi: 10.1086/517613. Epub 2007 Apr 17. J Infect Dis. 2007. PMID: 17471430
-
Mapping and analysis of West Nile virus-specific monoclonal antibodies: prospects for vaccine development.Expert Rev Vaccines. 2007 Apr;6(2):183-91. doi: 10.1586/14760584.6.2.183. Expert Rev Vaccines. 2007. PMID: 17408368 Review.
-
West Nile virus vaccines - current situation and future directions.Hum Vaccin Immunother. 2019;15(10):2337-2342. doi: 10.1080/21645515.2019.1621149. Epub 2019 Jul 10. Hum Vaccin Immunother. 2019. PMID: 31116691 Free PMC article. Review.
Cited by
-
The TLR4 agonist adjuvant SLA-SE promotes functional mucosal antibodies against a parenterally delivered ETEC vaccine.NPJ Vaccines. 2019 May 28;4:19. doi: 10.1038/s41541-019-0116-6. eCollection 2019. NPJ Vaccines. 2019. PMID: 31149350 Free PMC article.
-
Recombinant Zika Virus Subunits Are Immunogenic and Efficacious in Mice.mSphere. 2018 Jan 10;3(1):e00576-17. doi: 10.1128/mSphere.00576-17. eCollection 2018 Jan-Feb. mSphere. 2018. PMID: 29359186 Free PMC article.
-
GI-19007, a Novel Saccharomyces cerevisiae-Based Therapeutic Vaccine against Tuberculosis.Clin Vaccine Immunol. 2017 Dec 5;24(12):e00245-17. doi: 10.1128/CVI.00245-17. Print 2017 Dec. Clin Vaccine Immunol. 2017. PMID: 29046306 Free PMC article.
-
Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways To Promote Potent Adaptive Immune Responses.J Immunol. 2018 Jul 1;201(1):98-112. doi: 10.4049/jimmunol.1701604. Epub 2018 May 16. J Immunol. 2018. PMID: 29769270 Free PMC article.
-
Q Fever Vaccines: Unveiling the Historical Journey and Contemporary Innovations in Vaccine Development.Vaccines (Basel). 2025 Jan 31;13(2):151. doi: 10.3390/vaccines13020151. Vaccines (Basel). 2025. PMID: 40006698 Free PMC article. Review.
References
-
- Pepperell C, Rau N, Krajden S, Kern R, Humar A, Mederski B, et al. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in southcentral Ontario. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2003;168(11):1399–405. - PMC - PubMed
-
- Lindsey NP, Staples JE, Lehman JA, Fischer M, Centers for Disease C, Prevention. Surveillance for human West Nile virus disease—United States, 1999–2008. Morbidity and mortality weekly report Surveillance summaries. 2010;59(2):1–17. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

