Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus
- PMID: 26901180
- PMCID: PMC6273635
- DOI: 10.3390/molecules21020228
Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus
Abstract
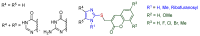
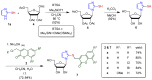
A series of new conjugated compounds with a -SCH₂- linkage were synthesized by chemical methods from imidazole and coumarin derivatives. The experimental results indicate that of the twenty newly synthesized imidazole-coumarin conjugates, three of them exhibited appealing EC50 values (5.1-8.4 μM) and selective indices >20 against hepatitis C virus. Their potency and selectivity were increased substantially by modification of their structure with two factors: imidazole nucleus with a hydrogen atom at the N(1) position and coumarin nucleus with a substituent, such as Cl, F, Br, Me, and OMe. These guidelines provide valuable information for further development of conjugated compounds as anti-viral agents.
Keywords: coumarin; hepatitis C virus; imidazole; nitrogen heterocycles; structure–activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Structure-activity relationship of new anti-hepatitis C virus agents: heterobicycle-coumarin conjugates.J Med Chem. 2009 Mar 12;52(5):1486-90. doi: 10.1021/jm801240d. J Med Chem. 2009. PMID: 19193060
-
Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus.Eur J Med Chem. 2013 May;63:290-8. doi: 10.1016/j.ejmech.2013.02.008. Epub 2013 Feb 19. Eur J Med Chem. 2013. PMID: 23501114
-
Synthesis of new benzimidazole-coumarin conjugates as anti-hepatitis C virus agents.Antiviral Res. 2008 Feb;77(2):157-62. doi: 10.1016/j.antiviral.2007.09.003. Epub 2007 Oct 8. Antiviral Res. 2008. PMID: 17977606
-
A review: Biologically active 3,4-heterocycle-fused coumarins.Eur J Med Chem. 2021 Feb 15;212:113034. doi: 10.1016/j.ejmech.2020.113034. Epub 2020 Nov 25. Eur J Med Chem. 2021. PMID: 33276991 Review.
-
Imidazole Derivatives as Potential Therapeutic Agents.Curr Pharm Des. 2016;22(21):3265-301. doi: 10.2174/1381612822666160226144333. Curr Pharm Des. 2016. PMID: 26916016 Review.
Cited by
-
Coumarin: An emerging antiviral agent.Heliyon. 2020 Jan 27;6(1):e03217. doi: 10.1016/j.heliyon.2020.e03217. eCollection 2020 Jan. Heliyon. 2020. PMID: 32042967 Free PMC article.
-
An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities.Int J Mol Sci. 2020 Jun 29;21(13):4618. doi: 10.3390/ijms21134618. Int J Mol Sci. 2020. PMID: 32610556 Free PMC article. Review.
-
Discovery of Bis-Imidazoline Derivatives as New CXCR4 Ligands.Molecules. 2023 Jan 24;28(3):1156. doi: 10.3390/molecules28031156. Molecules. 2023. PMID: 36770826 Free PMC article.
-
Transition metal-free oxidative and deoxygenative C-H/C-Li cross-couplings of 2H-imidazole 1-oxides with carboranyl lithium as an efficient synthetic approach to azaheterocyclic carboranes.Beilstein J Org Chem. 2018 Oct 12;14:2618-2626. doi: 10.3762/bjoc.14.240. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30410624 Free PMC article.
-
Synthesis of 3-(Imidazo[2,1-b]thiazol-6-yl)-2H-chromen-2-one Derivatives and Study of Their Antiviral Activity against Parvovirus B19.Molecules. 2019 Mar 15;24(6):1037. doi: 10.3390/molecules24061037. Molecules. 2019. PMID: 30875983 Free PMC article.
References
-
- Oze T., Hiramatsu N., Mita E., Akuta N., Sakamoto N., Nagano H., Itoh Y., Kaneko S., Izumi N., Nomura H., et al. A multicenter survey of re-treatment with pegylated interferon plus ribavirin combination therapy for patients with chronic hepatitis C in Japan. Hepatol. Res. 2013;43:35–43. doi: 10.1111/j.1872-034X.2012.01056.x. - DOI - PubMed
-
- Hulskotte E.G.J., Feng H.-P., Xuan F., Gupta S., van Zutven M.G.J.A., O’Mara E., Wagner J.A., Butterton J.R. Pharmacokinetic evaluation of the interaction between hepatitis C virus protease inhibitor boceprevir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and pravastatin. Antimicrob. Agents Chemother. 2013;57:2582–2588. doi: 10.1128/AAC.02347-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

