Multiplex PCR and Next Generation Sequencing for the Non-Invasive Detection of Bladder Cancer
- PMID: 26901314
- PMCID: PMC4762704
- DOI: 10.1371/journal.pone.0149756
Multiplex PCR and Next Generation Sequencing for the Non-Invasive Detection of Bladder Cancer
Abstract
Background: Highly sensitive and specific urine-based tests to detect either primary or recurrent bladder cancer have proved elusive to date. Our ever increasing knowledge of the genomic aberrations in bladder cancer should enable the development of such tests based on urinary DNA.
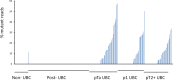
Methods: DNA was extracted from urine cell pellets and PCR used to amplify the regions of the TERT promoter and coding regions of FGFR3, PIK3CA, TP53, HRAS, KDM6A and RXRA which are frequently mutated in bladder cancer. The PCR products were barcoded, pooled and paired-end 2 x 250 bp sequencing performed on an Illumina MiSeq. Urinary DNA was analysed from 20 non-cancer controls, 120 primary bladder cancer patients (41 pTa, 40 pT1, 39 pT2+) and 91 bladder cancer patients post-TURBT (89 cancer-free).
Results: Despite the small quantities of DNA extracted from some urine cell pellets, 96% of the samples yielded mean read depths >500. Analysing only previously reported point mutations, TERT mutations were found in 55% of patients with bladder cancer (independent of stage), FGFR3 mutations in 30% of patients with bladder cancer, PIK3CA in 14% and TP53 mutations in 12% of patients with bladder cancer. Overall, these previously reported bladder cancer mutations were detected in 86 out of 122 bladder cancer patients (70% sensitivity) and in only 3 out of 109 patients with no detectable bladder cancer (97% specificity).
Conclusion: This simple, cost-effective approach could be used for the non-invasive surveillance of patients with non-muscle-invasive bladder cancers harbouring these mutations. The method has a low DNA input requirement and can detect low levels of mutant DNA in a large excess of normal DNA. These genes represent a minimal biomarker panel to which extra markers could be added to develop a highly sensitive diagnostic test for bladder cancer.
Conflict of interest statement
Figures



References
-
- van Kessel K, Van Neste L, Lurkin I, Zwarthoff E, Van Criekinge W (2015) Evaluation of an Epigenetic Profile for the Detection of Bladder Cancer in Hematuria Patients. J Urol epub ahead of print. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

