C-terminal Src Kinase Gates Homeostatic Synaptic Plasticity and Regulates Fasciclin II Expression at the Drosophila Neuromuscular Junction
- PMID: 26901416
- PMCID: PMC4764653
- DOI: 10.1371/journal.pgen.1005886
C-terminal Src Kinase Gates Homeostatic Synaptic Plasticity and Regulates Fasciclin II Expression at the Drosophila Neuromuscular Junction
Abstract
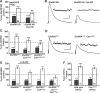
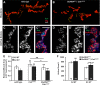
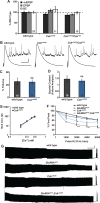
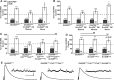
Forms of homeostatic plasticity stabilize neuronal outputs and promote physiologically favorable synapse function. A well-studied homeostatic system operates at the Drosophila melanogaster larval neuromuscular junction (NMJ). At the NMJ, impairment of postsynaptic glutamate receptor activity is offset by a compensatory increase in presynaptic neurotransmitter release. We aim to elucidate how this process operates on a molecular level and is preserved throughout development. In this study, we identified a tyrosine kinase-driven signaling system that sustains homeostatic control of NMJ function. We identified C-terminal Src Kinase (Csk) as a potential regulator of synaptic homeostasis through an RNAi- and electrophysiology-based genetic screen. We found that Csk loss-of-function mutations impaired the sustained expression of homeostatic plasticity at the NMJ, without drastically altering synapse growth or baseline neurotransmission. Muscle-specific overexpression of Src Family Kinase (SFK) substrates that are negatively regulated by Csk also impaired NMJ homeostasis. Surprisingly, we found that transgenic Csk-YFP can support homeostatic plasticity at the NMJ when expressed either in the muscle or in the nerve. However, only muscle-expressed Csk-YFP was able to localize to NMJ structures. By immunostaining, we found that Csk mutant NMJs had dysregulated expression of the Neural Cell Adhesion Molecule homolog Fasciclin II (FasII). By immunoblotting, we found that levels of a specific isoform of FasII were decreased in homeostatically challenged GluRIIA mutant animals-but markedly increased in Csk mutant animals. Additionally, we found that postsynaptic overexpression of FasII from its endogenous locus was sufficient to impair synaptic homeostasis, and genetically reducing FasII levels in Csk mutants fully restored synaptic homeostasis. Based on these data, we propose that Csk and its SFK substrates impinge upon homeostatic control of NMJ function by regulating downstream expression or localization of FasII.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

