Comparative Analysis of Technologies for Quantifying Extracellular Vesicles (EVs) in Clinical Cerebrospinal Fluids (CSF)
- PMID: 26901428
- PMCID: PMC4763994
- DOI: 10.1371/journal.pone.0149866
Comparative Analysis of Technologies for Quantifying Extracellular Vesicles (EVs) in Clinical Cerebrospinal Fluids (CSF)
Abstract
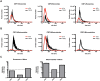
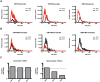
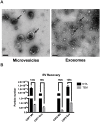
Extracellular vesicles (EVs) have emerged as a promising biomarker platform for glioblastoma patients. However, the optimal method for quantitative assessment of EVs in clinical bio-fluid remains a point of contention. Multiple high-resolution platforms for quantitative EV analysis have emerged, including methods grounded in diffraction measurement of Brownian motion (NTA), tunable resistive pulse sensing (TRPS), vesicle flow cytometry (VFC), and transmission electron microscopy (TEM). Here we compared quantitative EV assessment using cerebrospinal fluids derived from glioblastoma patients using these methods. For EVs <150 nm in diameter, NTA detected more EVs than TRPS in three of the four samples tested. VFC particle counts are consistently 2-3 fold lower than NTA and TRPS, suggesting contribution of protein aggregates or other non-lipid particles to particle count by these platforms. While TEM yield meaningful data in terms of the morphology, its particle count are consistently two orders of magnitude lower relative to counts generated by NTA and TRPS. For larger particles (>150 nm in diameter), NTA consistently detected lower number of EVs relative to TRPS. These results unveil the strength and pitfalls of each quantitative method alone for assessing EVs derived from clinical cerebrospinal fluids and suggest that thoughtful synthesis of multi-platform quantitation will be required to guide meaningful clinical investigations.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

