Pitchfork and Gprasp2 Target Smoothened to the Primary Cilium for Hedgehog Pathway Activation
- PMID: 26901434
- PMCID: PMC4763541
- DOI: 10.1371/journal.pone.0149477
Pitchfork and Gprasp2 Target Smoothened to the Primary Cilium for Hedgehog Pathway Activation
Abstract
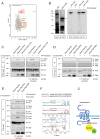
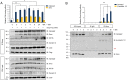
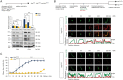
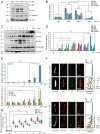
The seven-transmembrane receptor Smoothened (Smo) activates all Hedgehog (Hh) signaling by translocation into the primary cilia (PC), but how this is regulated is not well understood. Here we show that Pitchfork (Pifo) and the G protein-coupled receptor associated sorting protein 2 (Gprasp2) are essential components of an Hh induced ciliary targeting complex able to regulate Smo translocation to the PC. Depletion of Pifo or Gprasp2 leads to failure of Smo translocation to the PC and lack of Hh target gene activation. Together, our results identify a novel protein complex that is regulated by Hh signaling and required for Smo ciliary trafficking and Hh pathway activation.
Conflict of interest statement
Figures


References
-
- Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12(12):551–5. - PubMed
-
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–25. - PubMed
-
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell. 2007;129(7):1255–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

