National Variation in Use of Immunosuppression for Kidney Transplantation: A Call for Evidence-Based Regimen Selection
- PMID: 26901466
- PMCID: PMC5513703
- DOI: 10.1111/ajt.13758
National Variation in Use of Immunosuppression for Kidney Transplantation: A Call for Evidence-Based Regimen Selection
Abstract
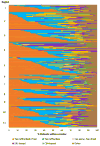
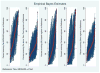
Immunosuppression management in kidney transplantation has evolved to include an increasingly diverse choice of medications. Although informed by patient and donor characteristics, choice of immunosuppression regimen varies widely across transplant programs. Using a novel database integrating national transplant registry and pharmacy fill records, immunosuppression use at 6-12 and 12-24 mo after transplant was evaluated for 22 453 patients transplanted in 249 U.S. programs in 2005-2010. Use of triple immunosuppression comprising tacrolimus, mycophenolic acid or azathioprine, and steroids varied widely (0-100% of patients per program), as did use of steroid-sparing regimens (0-77%), sirolimus-based regimens (0-100%) and cyclosporine-based regimens (0-78%). Use of triple therapy was more common in highly sensitized patients, women and recipients with dialysis duration >5 years. Sirolimus use appeared to diminish over the study period. Patient and donor characteristics explained only a limited amount of the observed variation in regimen use, whereas center choice explained 30-46% of the use of non-triple-therapy immunosuppression. The majority of patients who received triple-therapy (79%), cyclosporine-based (87.6%) and sirolimus-based (84.3%) regimens continued them in the second year after transplant. This population-based study of immunosuppression practice demonstrates substantial variation in center practice beyond that explained by differences in patient and donor characteristics.
Keywords: clinical research/practice; health services and outcomes research; immunosuppressant; immunosuppressive regimens; kidney transplantation/nephrology; maintenance; steroid.
© Copyright 2016 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Figures
References
-
- Lentine KL, Gheorghian A, Axelrod D, Kalsekar A, L’Italien G, Schnitzler MA. The implications of acute rejection for allograft survival in contemporary U.S. kidney transplantation. Transplantation. 2012;94(4):369–76. - PubMed
-
- Alangaden GJ, Thyagarajan R, Gruber SA, Morawski K, Garnick J, El-Amm JM, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20(4):401–9. - PubMed
-
- Pourmand G, Salem S, Mehrsai A, Taherimahmoudi M, Ebrahimi R, Pourmand M. Infectious complications after kidney transplantation: a single-center experience. Transplant Infectious Disease. 2007;9(4):302–9. - PubMed
-
- Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. Journal of the American Society of Nephrology. 2005;16(2):496–506. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

