High Resolution Topography of Age-Related Changes in Non-Rapid Eye Movement Sleep Electroencephalography
- PMID: 26901503
- PMCID: PMC4764685
- DOI: 10.1371/journal.pone.0149770
High Resolution Topography of Age-Related Changes in Non-Rapid Eye Movement Sleep Electroencephalography
Abstract
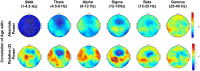
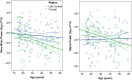
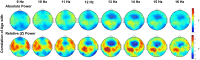
Sleeping brain activity reflects brain anatomy and physiology. The aim of this study was to use high density (256 channel) electroencephalography (EEG) during sleep to characterize topographic changes in sleep EEG power across normal aging, with high spatial resolution. Sleep was evaluated in 92 healthy adults aged 18-65 years old using full polysomnography and high density EEG. After artifact removal, spectral power density was calculated for standard frequency bands for all channels, averaged across the NREM periods of the first 3 sleep cycles. To quantify topographic changes with age, maps were generated of the Pearson's coefficient of the correlation between power and age at each electrode. Significant correlations were determined by statistical non-parametric mapping. Absolute slow wave power declined significantly with increasing age across the entire scalp, whereas declines in theta and sigma power were significant only in frontal regions. Power in fast spindle frequencies declined significantly with increasing age frontally, whereas absolute power of slow spindle frequencies showed no significant change with age. When EEG power was normalized across the scalp, a left centro-parietal region showed significantly less age-related decline in power than the rest of the scalp. This partial preservation was particularly significant in the slow wave and sigma bands. The effect of age on sleep EEG varies substantially by region and frequency band. This non-uniformity should inform the design of future investigations of aging and sleep. This study provides normative data on the effect of age on sleep EEG topography, and provides a basis from which to explore the mechanisms of normal aging as well as neurodegenerative disorders for which age is a risk factor.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

