Neonatal thymectomy reveals differentiation and plasticity within human naive T cells
- PMID: 26901814
- PMCID: PMC4767338
- DOI: 10.1172/JCI84997
Neonatal thymectomy reveals differentiation and plasticity within human naive T cells
Abstract
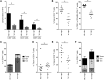
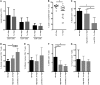
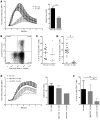
The generation of naive T cells is dependent on thymic output, but in adults, the naive T cell pool is primarily maintained by peripheral proliferation. Naive T cells have long been regarded as relatively quiescent cells; however, it was recently shown that IL-8 production is a signatory effector function of naive T cells, at least in newborns. How this functional signature relates to naive T cell dynamics and aging is unknown. Using a cohort of children and adolescents who underwent neonatal thymectomy, we demonstrate that the naive CD4+ T cell compartment in healthy humans is functionally heterogeneous and that this functional diversity is lost after neonatal thymectomy. Thymic tissue regeneration later in life resulted in functional restoration of the naive T cell compartment, implicating the thymus as having functional regenerative capacity. Together, these data shed further light on functional differentiation within the naive T cell compartment and the importance of the thymus in human naive T cell homeostasis and premature aging. In addition, these results affect and alter our current understanding on the identification of truly naive T cells and recent thymic emigrants.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

