Teriparatide for osteoporosis: importance of the full course
- PMID: 26902094
- PMCID: PMC4947115
- DOI: 10.1007/s00198-016-3534-6
Teriparatide for osteoporosis: importance of the full course
Abstract
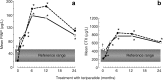
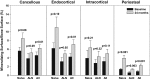
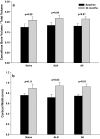
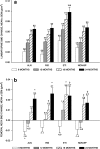
Teriparatide (TPTD) is the only currently available therapeutic agent that increases the formation of new bone tissue and can provide some remediation of the architectural defects in the osteoporotic skeleton. The use of teriparatide clinically is limited to 24 months. We review clinical findings during daily teriparatide treatment over time. Teriparatide appears to increase bone formation more than bone resorption as determined biochemically and histologically. Teriparatide exerts its positive effects on bone formation in two distinct fashions. The first is direct stimulation of bone formation that occurs within active remodeling sites (remodeling-based bone formation) and on surfaces of bone previously inactive (modeling-based bone formation). The second is an increase in the initiation of new remodeling sites. Both processes contribute to the final increase in bone density observed by non-invasive tools such as DXA. Remodeling is the repair process by which skeletal tissue is maintained in a young healthy state, and when stimulated by TPTD is associated with a positive bone balance within each remodeling cavity. It seems likely therefore that this component will contribute to the anti-fracture efficacy of TPTD. Teriparatide reduces the risk of fracture, and this effect appears to increase with longer duration of therapy. The use of novel treatment regimens, including shorter courses, should be held in abeyance until controlled clinical trials are completed to define the relative fracture benefits of such approaches in comparison to the 24-month daily use of the agent. Summary In patients with osteoporosis at high risk for fracture, the full continuous 24-month course with teriparatide results in improved skeletal health and outcomes than shorter time periods.
Keywords: Bone histomorphometry; Fracture; Osteoporosis; Teriparatide.
Figures


Comment in
-
Full 24-month treatment course with daily teriparatide: a mechanistic insight.Osteoporos Int. 2016 Aug;27(8):2635-6. doi: 10.1007/s00198-016-3630-7. Epub 2016 May 6. Osteoporos Int. 2016. PMID: 27154436 No abstract available.
References
-
- Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703. doi: 10.1210/er.2004-0006. - DOI - PubMed
-
- FORTEO [full prescribing information]. Eli Lilly and Company, Indianapolis, IN;March 2012. Available at: http://pi.lilly.com/us/foreo-pi.pdf. Accessed September 29, 2015
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. doi: 10.1056/NEJM200105103441904. - DOI - PubMed
-
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312–321. doi: 10.1080/01926230252929882. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

