Control of Foxp3 stability through modulation of TET activity
- PMID: 26903244
- PMCID: PMC4813667
- DOI: 10.1084/jem.20151438
Control of Foxp3 stability through modulation of TET activity
Abstract
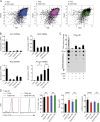
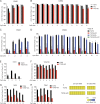
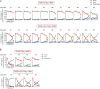
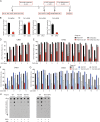
Ten-eleven translocation (TET) enzymes oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine and other oxidized methylcytosines, intermediates in DNA demethylation. In this study, we examine the role of TET proteins in regulating Foxp3, a transcription factor essential for the development and function of regulatory T cells (T reg cells), a distinct lineage of CD4(+) T cells that prevent autoimmunity and maintain immune homeostasis. We show that during T reg cell development in the thymus, TET proteins mediate the loss of 5mC in T reg cell-specific hypomethylated regions, including CNS1 and CNS2, intronic cis-regulatory elements in the Foxp3 locus. Similar to CNS2-deficient T reg cells, the stability of Foxp3 expression is markedly compromised in T reg cells from Tet2/Tet3 double-deficient mice. Vitamin C potentiates TET activity and acts through Tet2/Tet3 to increase the stability of Foxp3 expression in TGF-β-induced T reg cells. Our data suggest that targeting TET enzymes with small molecule activators such as vitamin C might increase induced T reg cell efficacy.
© 2016 Yue et al.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

