Statin adjunctive therapy shortens the duration of TB treatment in mice
- PMID: 26903278
- PMCID: PMC5007636
- DOI: 10.1093/jac/dkw014
Statin adjunctive therapy shortens the duration of TB treatment in mice
Abstract
Background: The repurposing of existing agents may accelerate TB drug development. Recently, we reported that the lipid-lowering drug simvastatin, when added to the first-line antitubercular regimen, reduces the lung bacillary burden in chronically infected mice.
Objectives: We investigated whether the addition of simvastatin to the first-line regimen (isoniazid/rifampicin/pyrazinamide) shortens the duration of curative TB treatment in mice.
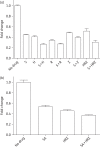
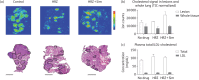
Methods: Mycobacterium tuberculosis-infected THP-1 cells were exposed to simvastatin to determine the effect of statins on the activity of first-line anti-TB drug activity and intracellular rifampicin concentration. Single-dose and steady-state pharmacokinetic studies guided optimized simvastatin dosing in vivo. BALB/c mice were aerosol-infected with M. tuberculosis H37Rv and drug treatment was initiated 6 weeks post-infection. Separate groups of mice received standard TB treatment with or without simvastatin. Relapse rates were assessed 3 months after discontinuation of each treatment regimen. MALDI-MS imaging was used to image the cholesterol content of mouse lung lesions.
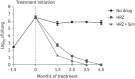
Results: Simvastatin significantly enhanced the bactericidal activity of first-line drugs against intracellular M. tuberculosis without altering intracellular rifampicin concentrations. Adjunctive treatment with 60 mg/kg simvastatin shortened the time required to achieve culture-negative lungs from 4.5 to 3.5 months. Following 2.5, 3.5 and 4.5 months of treatment, relapse rates were 100%, 50% and 0%, respectively, in the control group and 50% (P = 0.03), 20% and 0%, respectively, in the statin group. Simvastatin did not alter plasma or lung lesion cholesterol levels.
Conclusions: Statins are attractive candidates for host-directed, adjunctive TB therapy. Further preclinical studies are needed to define the optimal statin and dosing.
© The Author 2016. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Zumla AI, Gillespie SH, Hoelscher M et al. . New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis 2014; 14: 327–40. - PubMed
-
- Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 2015; 15: 255–63. - PubMed
-
- Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med 2002; 346: 539–40. - PubMed
-
- Davidson MH, Robinson JG. Lipid-lowering effects of statins: a comparative review. Expert Opin Pharmacother 2006; 7: 1701–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

