Grainyhead-like 2 downstream targets act to suppress epithelial-to-mesenchymal transition during neural tube closure
- PMID: 26903501
- PMCID: PMC4852492
- DOI: 10.1242/dev.129825
Grainyhead-like 2 downstream targets act to suppress epithelial-to-mesenchymal transition during neural tube closure
Abstract
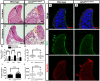
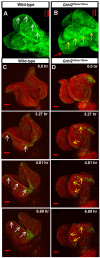
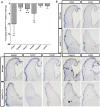
The transcription factor grainyhead-like 2 (GRHL2) is expressed in non-neural ectoderm (NNE) and Grhl2 loss results in fully penetrant cranial neural tube defects (NTDs) in mice. GRHL2 activates expression of several epithelial genes; however, additional molecular targets and functional processes regulated by GRHL2 in the NNE remain to be determined, as well as the underlying cause of the NTDs in Grhl2 mutants. Here, we find that Grhl2 loss results in abnormal mesenchymal phenotypes in the NNE, including aberrant vimentin expression and increased cellular dynamics that affects the NNE and neural crest cells. The resulting loss of NNE integrity contributes to an inability of the cranial neural folds to move toward the midline and results in NTD. Further, we identified Esrp1, Sostdc1, Fermt1, Tmprss2 and Lamc2 as novel NNE-expressed genes that are downregulated in Grhl2 mutants. Our in vitro assays show that they act as suppressors of the epithelial-to-mesenchymal transition (EMT). Thus, GRHL2 promotes the epithelial nature of the NNE during the dynamic events of neural tube formation by both activating key epithelial genes and actively suppressing EMT through novel downstream EMT suppressors.
Keywords: Epithelial-to-mesenchymal transition; Grhl2; Mouse; Neural crest cells; Neural tube closure; Non-neural ectoderm; Trim29.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





References
-
- Aue A., Hinze C., Walentin K., Ruffert J., Yurtdas Y., Werth M., Chen W., Rabien A., Kilic E., Schulzke J.-D. et al. (2015). A Grainyhead-Like 2/Ovo-Like 2 pathway regulates renal epithelial barrier function and lumen expansion. J. Am. Soc. Nephrol. 26, 2704-2715. 10.1681/asn.2014080759 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

